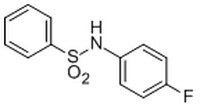
530583 Sigma-Aldrichα-Synuclein Blocker, ELN484228 - CAS 312-63-0 - Calbiochem
A cell-permeable compound that reversibly binds to pocket I of the monomeric α-synuclein (α-syn) and reverses α-syn-induced impairment of phagocytosis in H4 neuroglioma cells.
More>> A cell-permeable compound that reversibly binds to pocket I of the monomeric α-synuclein (α-syn) and reverses α-syn-induced impairment of phagocytosis in H4 neuroglioma cells. Less<<Synonyms: N-(4-Fluorophenyl)benzenesulfonamide, NSC164389, ELN 484228
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 312-63-0 | C₁₂H₁₀FNO₂S |
Products
| Catalog Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.30583.0001 | Glass bottle | 25 mg |
| References | |
|---|---|
| References | Toth, G., et al. 2014. PLoS ONE 9, e87133. |
| Product Information | |
|---|---|
| CAS number | 312-63-0 |
| Form | Off-white solid |
| Hill Formula | C₁₂H₁₀FNO₂S |
| Chemical formula | C₁₂H₁₀FNO₂S |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | monomeric α-synuclein |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 5.30583.0001 | 04055977260915 |
Documentation
α-Synuclein Blocker, ELN484228 - CAS 312-63-0 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Toth, G., et al. 2014. PLoS ONE 9, e87133. |