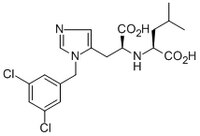
530616 Sigma-AldrichACE2 Inhibitor, MLN-4760 - CAS 305335-31-3 - Calbiochem
A cell-permeable, highly potent inhibitor of angiotensin converting enzyme 2 (ACE2; IC₅₀ = 440 pM).
More>> A cell-permeable, highly potent inhibitor of angiotensin converting enzyme 2 (ACE2; IC₅₀ = 440 pM). Less<<Synonyms: (S,S)-2-(1-Carboxy-2-(3-(3,5-dichlorobenzyl)-3H-imidazol-4-yl)-ethylamino)-4-methylpentanoic acid, 2(S)-(1(S)-Carboxy-2-(3-(3,5-dichlorobenzyl)-3H-imidazol-4-yl)-ethylamino)-4-methylpentanoic acid, Angiotensin-Converting Enzyme-Related Carboxypeptidase Inhibitor, ML00106791, GL-1001, GL1001, MLN4760
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 305335-31-3 | C₁₉H₂₃Cl₂N₃O₄ |
Products
| Catalog Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.30616.0001 | Glass bottle | 2 mg |
| Product Information | |
|---|---|
| CAS number | 305335-31-3 |
| Form | White powder |
| Hill Formula | C₁₉H₂₃Cl₂N₃O₄ |
| Chemical formula | C₁₉H₂₃Cl₂N₃O₄ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | soluble human ACE2 |
| Primary Target IC<sub>50</sub> | 440 pM for soluble human ACE2 |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 5.30616.0001 | 04055977260960 |
Documentation
ACE2 Inhibitor, MLN-4760 - CAS 305335-31-3 - Calbiochem SDS
| Title |
|---|
ACE2 Inhibitor, MLN-4760 - CAS 305335-31-3 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 530616 |
References
| Reference overview |
|---|
| Tikellis, C., et al. 2014. Am. J. Physiol. Renal Physiol. 306, F773. Grobe, N., et al. 2013. Am. J. Physiol. Cell Physiol 304, C945. Ye, M., et al. 2012. Hypertension 60, 730. Dilauro, M., et al. 2010. Am. J. Physiol. Renal Physiol. 298, F1523. Thomas, M.C., et al. 2010. Circ. Res. 107, 888. Trask, A.J., et al. 2010. Am. J. Hypertens. 23, 687. Byrnes, J.J., et al. 2009. Inflamm. Res. 58, 819. Soler, M.J., et al. 2007. Kidney Int. 72, 614. Towler, P., et al. 2004. J. Biol. Chem. 279, 17996. Dales, N.A., et al. 2002. J. Am. Chem. Soc. 124, 11852. |