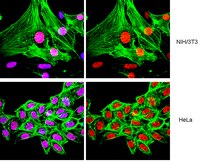
The malate-aspartate NADH shuttle member Aralar1 determines glucose metabolic fate, mitochondrial activity, and insulin secretion in beta cells.
Blanca Rubi,Araceli del Arco,Clarissa Bartley,Jorgina Satrustegui,Pierre Maechler
The Journal of biological chemistry
279
2004
Show Abstract
The NADH shuttle system, which transports reducing equivalents from the cytosol to the mitochondria, is essential for the coupling of glucose metabolism to insulin secretion in pancreatic beta cells. Aralar1 and citrin are two isoforms of the mitochondrial aspartate/glutamate carrier, one key constituent of the malate-aspartate NADH shuttle. Here, the effects of Aralar1 overexpression in INS-1E beta cells and isolated rat islets were investigated for the first time. We prepared a recombinant adenovirus encoding for human Aralar1 (AdCA-Aralar1), tagged with the small FLAG epitope. Transduction of INS-1E cells and isolated rat islets with AdCA-Aralar1 increased aralar1 protein levels and immunostaining revealed mitochondrial localization. Compared with control INS-1E cells, overexpression of Aralar1 potentiated metabolism secretion coupling stimulated by 15 mm glucose. In particular, there was an increase of NAD(P)H generation, of mitochondrial membrane hyperpolarization, ATP levels, glucose oxidation, and insulin secretion (+45%, p < 0.01). Remarkably, this was accompanied by reduced lactate production. Rat islets overexpressing Aralar1 secreted more insulin at 16.7 mm glucose (+65%, p < 0.05) compared with controls. These results show that aspartate-glutamate carrier capacity limits glucose-stimulated insulin secretion and that Aralar1 overexpression enhances mitochondrial metabolism. | 15494407
 |
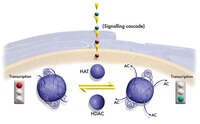
The TAF(II)250 subunit of TFIID has histone acetyltransferase activity.
Mizzen, C A, et al.
Cell, 87: 1261-70 (1996)
1996
Show Abstract
The transcription initiation factor TFIID is a multimeric protein complex composed of TATA box-binding protein (TBP) and many TBP-associated factors (TAF(II)s). TAF(II)s are important cofactors that mediate activated transcription by providing interaction sites for distinct activators. Here, we present evidence that human TAF(II)250 and its homologs in Drosophila and yeast have histone acetyltransferase (HAT) activity in vitro. HAT activity maps to the central, most conserved portion of dTAF(II)230 and yTAF(II)130. The HAT activity of dTAF(II)230 resembles that of yeast and human GCN5 in that it is specific for histones H3 and H4 in vitro. Our findings suggest that targeted histone acetylation at specific promoters by TAF(II)250 may be involved in mechanisms by which TFIID gains access to transcriptionally repressed chromatin. | 8980232
 |
Mutagenic effects of niridazole in animal-mediated and in liquid suspension assays using Escherichia coli K-12 as an indicator.
S Knasmüller,A Szakmary
Mutation research
280
1992
Show Abstract
The mutagenic effects of the antischistosomal drug niridazole (1-(5-nitro-2-thiazolyl)-2-imidazolidinone) were investigated in liquid suspension and intrasanguineous animal-mediated assays with mice. As indicator strains Escherichia coli K-12 343/113 (Nir(S)) and a newly constructed niridazole nitroreductase-deficient derivative (Escherichia coli K-12 343/113 Nir(r) 200) were used. With the parental strain (Nir(S)) induction of nalidixic acid- and valine-resistant mutants was observed under in vivo conditions in the liver and, to a lesser extent, in the spleen. Positive results were also found when intestinal homogenates, blood sera, and urine samples of niridazole-treated animals were tested in vitro with the wild-type strain. With Escherichia coli K-12 343/113 Nir(r) 200 no clear-cut positive results were obtained in animal-mediated assays. In liquid suspension assays positive results were restricted to the urine samples. These findings indicate that the positive results obtained with the wild-type strain are due to nitroreduction and that the concentrations of mutagenic metabolites formed by activation processes in the living animal are too low to enable their detection in inner organs, intestines, and the blood with the reductase-deficient strain. In agreement with our present findings showing increased genotoxicity in urine, niridazole causes tumors in rodents preferentially in the kidneys and in the bladder. | 1378543
 |