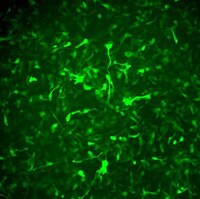
SCR526 Sigma-AldrichAlzheimer’s In A Dish™ APPSL-GFP Lentivirus
The APPSL-GFP lentivirus can be used to generate 3D cell culture models of Alzheimer’s Disease. The high-titer polycistronic lentiviral particles contain the APPSL and TagGFP2 proteins and can be used to infect human neural stem cells at high efficiency.
More>> The APPSL-GFP lentivirus can be used to generate 3D cell culture models of Alzheimer’s Disease. The high-titer polycistronic lentiviral particles contain the APPSL and TagGFP2 proteins and can be used to infect human neural stem cells at high efficiency. Less<<Recommended Products
Overview
| Replacement Information |
|---|
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Species Reactivity |
|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements | |
|---|---|
| Quality Assurance | The packaged lentivector constructs are provided as frozen VSV-G pseudotyped viral particles. The titer (ifus/ml) is defined by SBI’s Global UltraRapid Lentiviral Titer Kit (Cat. # LV961A-1) in HT1080 cells transduced by the packaged constructs. |
| Usage Statement |
|
| Packaging Information | |
|---|---|
| Material Size | 15ul/vial |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| SCR526 | 04054839338649 |
Documentation
Alzheimer’s In A Dish™ APPSL-GFP Lentivirus SDS
| Title |
|---|
User Guides
| Title |
|---|
| APPSL-GFP Alzheimer¿s Lentivirus |