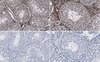
Reelin immunoreactivity in neuritic varicosities in the human hippocampal formation of non-demented subjects and Alzheimer's disease patients.
Notter, T; Knuesel, I
Acta neuropathologica communications
1
27
2013
Show Abstract
Reelin and its downstream signaling members are important modulators of actin and microtubule cytoskeleton dynamics, a fundamental prerequisite for proper neurodevelopment and adult neuronal functions. Reductions in Reelin levels have been suggested to contribute to Alzheimer's disease (AD) pathophysiology. We have previously reported an age-related reduction in Reelin levels and its accumulation in neuritic varicosities along the olfactory-limbic tracts, which correlated with cognitive impairments in aged mice. Here, we aimed to investigate whether a similar Reelin-associated neuropathology is observed in the aged human hippocampus and whether it correlated with dementia status.Our immunohistochemical stainings revealed the presence of N- and C-terminus-containing Reelin fragments in corpora amylacea (CAm), aging-associated spherical deposits. The density of these deposits was increased in the molecular layer of the subiculum of AD compared to non-demented individuals. Despite the limitation of a small sample size, our evaluation of several neuronal and glial markers indicates that the presence of Reelin in CAm might be related to aging-associated impairments in neuronal transport leading to accumulation of organelles and protein metabolites in neuritic varicosities, as previously suggested by the findings and discussions in rodents and primates.Our results indicate that aging- and disease-associated changes in Reelin levels and proteolytic processing might play a role in the formation of CAm by altering cytoskeletal dynamics. However, its presence may also be an indicator of a degenerative state of neuritic compartments. | | | 24252415
 |
Exercise during pregnancy mitigates Alzheimer-like pathology in mouse offspring.
Herring, A; Donath, A; Yarmolenko, M; Uslar, E; Conzen, C; Kanakis, D; Bosma, C; Worm, K; Paulus, W; Keyvani, K
FASEB journal : official publication of the Federation of American Societies for Experimental Biology
26
117-28
2012
Show Abstract
Physical activity protects brain function in healthy individuals and those with Alzheimer's disease (AD). Evidence for beneficial effects of parental exercise on the health status of their progeny is sparse and limited to nondiseased individuals. Here, we questioned whether maternal running interferes with offspring's AD-like pathology and sought to decipher the underlying mechanisms in TgCRND8 mice. Maternal stimulation was provided by voluntary wheel running vs. standard housing during pregnancy. Following 5 mo of standard housing of transgenic and wild-type offspring, their brains were examined for AD-related pathology and/or plasticity changes. Running during pregnancy reduced β-amyloid (Aβ) plaque burden (-35%, P=0.017) and amyloidogenic APP processing in transgenic offspring and further improved the neurovascular function by orchestrating different Aβ transporters and increasing angiogenesis (+29%, P=0.022). This effect was accompanied by diminished inflammation, as indicated by reduced microgliosis (-20%, P=0.002) and down-regulation of other proinflammatory mediators, and resulted in less oxidative stress, as nitrotyrosine levels declined (-28%, P=0.029). Moreover, plasticity changes (in terms of up-regulation of reelin, synaptophysin, and ARC) were found not only in transgenic but also in wild-type offspring. We conclude that exercise during pregnancy provides long-lasting protection from neurodegeneration and improves brain plasticity in the otherwise unstimulated progeny. | Immunohistochemistry | | 21948247
 |
Probucol suppresses enterocytic accumulation of amyloid-β induced by saturated fat and cholesterol feeding.
Menuka M Pallebage-Gamarallage,Susan Galloway,Ryusuke Takechi,Satvinder Dhaliwal,John C L Mamo
Lipids
47
2012
Show Abstract
Amyloid-β (Aβ) is secreted from lipogenic organs such as intestine and liver as an apolipoprotein of nascent triacylglycerol rich lipoproteins. Chronically elevated plasma Aβ may compromise cerebrovascular integrity and exacerbate amyloidosis--a hallmark feature of Alzheimer's disease (AD). Probucol is a hypocholesterolemic agent that reduces amyloid burden in transgenic amyloid mice, but the mechanisms for this effect are presently unclear. In this study, the effect of Probucol on intestinal lipoprotein-Aβ homeostasis was explored. Wild-type mice were fed a control low-fat diet and enterocytic Aβ was stimulated by high-fat (HF) diet enriched in 10% (w/w) saturated fat and 1% (w/w) cholesterol for the duration of 1 month. Mice treated with Probucol had the drug incorporated into the chow at 1% (w/w). Quantitative immunofluorescence was utilised to determine intestinal apolipoprotein B (apo B) and Aβ abundance. We found apo B in both the perinuclear region of the enterocytes and the lacteals in all groups. However, HF feeding and Probucol treatment increased secretion of apo B into the lacteals without any change in net villi abundance. On the other hand, HF-induced enterocytic perinuclear Aβ was significantly attenuated by Probucol. No significant changes in Aβ were observed within the lacteals. The findings of this study support the notion that Probucol suppresses dietary fat induced stimulation of Aβ biosynthesis and attenuate availability of apo B lipoprotein-Aβ for secretion. | | | 21805327
 |
Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice.
Krstic, D; Madhusudan, A; Doehner, J; Vogel, P; Notter, T; Imhof, C; Manalastas, A; Hilfiker, M; Pfister, S; Schwerdel, C; Riether, C; Meyer, U; Knuesel, I
Journal of neuroinflammation
9
151
2012
Show Abstract
Alzheimer's disease (AD) is the most prevalent form of age-related dementia, and its effect on society increases exponentially as the population ages. Accumulating evidence suggests that neuroinflammation, mediated by the brain's innate immune system, contributes to AD neuropathology and exacerbates the course of the disease. However, there is no experimental evidence for a causal link between systemic inflammation or neuroinflammation and the onset of the disease.The viral mimic, polyriboinosinic-polyribocytidilic acid (PolyI:C) was used to stimulate the immune system of experimental animals. Wild-type (WT) and transgenic mice were exposed to this cytokine inducer prenatally (gestation day (GD)17) and/or in adulthood. Behavioral, immunological, immunohistochemical, and biochemical analyses of AD-associated neuropathologic changes were performed during aging.We found that a systemic immune challenge during late gestation predisposes WT mice to develop AD-like neuropathology during the course of aging. They display chronic elevation of inflammatory cytokines, an increase in the levels of hippocampal amyloid precursor protein (APP) and its proteolytic fragments, altered Tau phosphorylation, and mis-sorting to somatodendritic compartments, and significant impairments in working memory in old age. If this prenatal infection is followed by a second immune challenge in adulthood, the phenotype is strongly exacerbated, and mimics AD-like neuropathologic changes. These include deposition of APP and its proteolytic fragments, along with Tau aggregation, microglia activation and reactive gliosis. Whereas Aβ peptides were not significantly enriched in extracellular deposits of double immune-challenged WT mice at 15 months, they dramatically increased in age-matched immune-challenged transgenic AD mice, precisely around the inflammation-induced accumulations of APP and its proteolytic fragments, in striking similarity to the post-mortem findings in human patients with AD.Chronic inflammatory conditions induce age-associated development of an AD-like phenotype in WT mice, including the induction of APP accumulations, which represent a seed for deposition of aggregation-prone peptides. The PolyI:C mouse model therefore provides a unique tool to investigate the molecular mechanisms underlying the earliest pathophysiological changes preceding fibrillary Aβ plaque deposition and neurofibrillary tangle formations in a physiological context of aging. Based on the similarity between the changes in immune-challenged mice and the development of AD in humans, we suggest that systemic infections represent a major risk factor for the development of AD. | Immunoblotting (Western) | Mouse | 22747753
 |
COL25A1 triggers and promotes Alzheimer's disease-like pathology in vivo.
Tong, Y; Xu, Y; Scearce-Levie, K; Ptácek, LJ; Fu, YH
Neurogenetics
11
41-52
2010
Show Abstract
Collagen XXV alpha 1 (COL25A1) is a collagenous type II transmembrane protein purified from senile plaques of Alzheimer's disease (AD) brains. COL25A1 alleles have been associated with increased risk for AD in a Swedish population. COL25A1 is specifically expressed in neurons and binds to aggregated Abeta in vitro. However, its contribution to the pathogenesis of AD and in vivo function are unknown. Here, we report that over-expression of COL25A1 in transgenic mice increases p35/p25 and beta-site APP-cleaving enzyme 1 (BACE1) levels, facilitates intracellular aggregation and extracellular matrix deposits of Abeta, and causes synaptophysin loss and astrocyte activation. COL25A1 mice displayed reduced anxiety-like behavior in elevated plus maze and open field tests and significantly slower swimming speed in Morris water maze. In stable cell lines, motifs in noncollagenous domains of COL25A1 were important for the induction of BACE1 expression. These findings demonstrate that COL25A1 leads to AD-like pathology in vivo. Modulation of COL25A1 function may represent an alternative therapeutic intervention for AD. Full Text Article | Immunohistochemistry | | 19548013
 |
The effect of exogenous cholesterol and lipid-modulating agents on enterocytic amyloid-beta abundance.
Pallebage-Gamarallage, MM; Galloway, S; Johnsen, R; Jian, L; Dhaliwal, S; Mamo, JC
The British journal of nutrition
101
340-7
2009
Show Abstract
Dietary cholesterol may influence Alzheimer's disease risk, because it regulates the synthesis of amyloid-beta. It was recently demonstrated in enterocytes of wild-type mice that intracellular amyloid-beta expression is enhanced in response to a high-fat diet made up of SFA and cholesterol. Intestinally derived amyloid-beta may be associated with postprandial lipoproteins in response to dietary fats and could be a key regulator in chylomicron metabolism. The present study was designed to investigate the role of cholesterol in modulating amyloid-beta abundance in enterocytes. Wild-type mice were fed a low-fat diet supplemented with 2 % (w/w) cholesterol. The effects of cholesterol absorption inhibition and cholesterol biosynthesis inhibition utilising ezetimibe and atorvastatin, respectively, were also studied. Quantitative immunohistochemistry was utilised to determine enterocytic amyloid-beta homeostasis. We found that enterocytic amyloid-beta concentration was significantly attenuated in mice fed the 2 % (w/w) cholesterol diet. However, blocking cholesterol absorption reversed the cholesterol-feeding effect. Consistent with a suppressive effect of cholesterol on enterocytic amyloid-beta abundance, atorvastatin, an inhibitor of cholesterol biosynthesis, enhanced amyloid-beta. However, providing exogenous cholesterol abolished the atorvastatin-induced effect. In contrast to the suppression of enterocytic amyloid-beta by dietary cholesterol, mice fed a diet enriched in SFA had markedly greater abundance. Collectively, the findings suggest that exogenous and endogenous cholesterol reduce amyloid-beta concentration in enterocytes by suppressing production, or enhancing secretion associated with postprandial lipoproteins. Intestinally derived amyloid-beta will contribute to the pool of plasma protein and may influence cerebral amyloid homeostasis by altering the bi-directional transfer across the blood-brain barrier. | | | 18631412
 |
Synergistic effects of high fat feeding and apolipoprotein E deletion on enterocytic amyloid-beta abundance.
Galloway, S; Pallebage-Gamarallage, MM; Takechi, R; Jian, L; Johnsen, RD; Dhaliwal, SS; Mamo, JC
Lipids in health and disease
7
15
2008
Show Abstract
Amyloid-beta (Abeta), a key protein found in amyloid plaques of subjects with Alzheimer's disease is expressed in the absorptive epithelial cells of the small intestine. Ingestion of saturated fat significantly enhances enterocytic Abeta abundance whereas fasting abolishes expression. Apolipoprotein (apo) E has been shown to directly modulate Abeta biogenesis in liver and neuronal cells but it's effect in enterocytes is not known. In addition, apo E modulates villi length, which may indirectly modulate Abeta as a consequence of differences in lipid absorption. This study compared Abeta abundance and villi length in wild-type (WT) and apo E knockout (KO) mice maintained on either a low-fat or high-fat diet. Wild-type C57BL/6J and apo E KO mice were randomised for six-months to a diet containing either 4% (w/w) unsaturated fats, or chow comprising 16% saturated fats and 1% cholesterol. Quantitative immunohistochemistry was used to assess Abeta abundance in small intestinal enterocytes. Apo E KO mice given the low-fat diet had similar enterocytic Abeta abundance compared to WT controls.The saturated fat diet substantially increased enterocytic Abeta in WT and in apo E KO mice, however the effect was greater in the latter. Villi height was significantly greater in apo E KO mice than for WT controls when given the low-fat diet. However, WT mice had comparable villi length to apo E KO when fed the saturated fat and cholesterol enriched diet. There was no effect of the high-fat diet on villi length in apo E KO mice.The findings of this study are consistent with the notion that lipid substrate availability modulates enterocytic Abeta. Apo E may influence enterocytic lipid availability by modulating absorptive capacity. | | | 18426603
 |
Genetic loci contributing to age-related hippocampal lesions in mice.
Kelly L Krass, Veronica Colinayo, Anatole Ghazalpour, Harry V Vinters, Aldons J Lusis, Thomas A Drake
Neurobiology of disease
13
102-8
2003
Show Abstract
C57BL/6J mice develop genetically determined age-related hippocampal granular deposits that have some similarities to lesions seen in the brains of human patients with tau protein related neurodegenerative disorders (tauopathies). We sought to identify the genetic loci responsible for these in an F2 intercross of inbred mouse strains C57BL/6J and DBA/2J, using quantitative trait locus (QTL) analysis. Hippocampal lesions were shown to be PAS positive, H and E negative, and immunoreactive for tau protein and alpha synuclein, but not to Abeta 1-40 or Abeta 1-42, or for ubiquitin. These were quantitated by histomorphometry, and QTL analysis revealed a locus on chromosome 7 with a lod score of 6.5 as well as two suggestive loci on chromosome 10. The genomic data indicate that the genetic basis is complex, but with one locus playing a major role in lesion formation. These lesions may represent a useful model for investigating dysregulation of tau protein in the hippocampus. | | | 12828934
 |