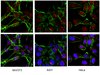
Sexual differences in cell proliferation in the ventricular zone, cell migration and differentiation in the HVC of juvenile Bengalese finch.
Chen, Q; Zhang, X; Zhao, Y; Zhou, X; Sun, L; Zeng, S; Zuo, M; Zhang, X
PloS one
9
e97403
2014
Show Abstract
Song control nuclei have distinct sexual differences and thus are an ideal model to address how brain areas are sexually differentiated. Through a combination of histological analysis and electrical lesions, we first identified the ventricle site for HVC progenitor cells. We then found that there were significant sex differences in the cellular proliferation activity in the ventricular zone of the HVC, the number of migrating cells along the radial cells (positive immunoreactions to vimentin) and differentiation towards neurons. Through co-culturing of male and female slices containing the developing HVC in the same well, we found that the male slices could produce diffusible substances to masculinize the female HVC. By adding estrogen, an estrogen antagonist, brain-derived neurotrophic factor (BDNF) or its antibody into the culture medium, separately or in combination, we found that these diffusible substances may include estrogen and BDNF. Finally, we found that 1) estrogen-induced BDNF upregulation could be detected 48 hr after estrogen treatment and could not be blocked by a vascular endothelial growth factor (VEGF) receptor inhibitor and 2) the amount of VEGF mRNA expressed in the developing HVC and its adjacent area did not display any significant sex differences, as did the distribution of VEGF and laminin-expressing endothelial cells in the developing HVC. Because these findings are largely different from previous reports on the adult female HVC, it is suggested that our estrogen-induced BDNF up-regulation and the resultant sexual differentiation might not be mediated by VEGF and endothelial cells, but instead, may result from the direct effects of estrogen on BDNF. | 24841082
 |
Hippocampal--prefrontal BDNF and memory for fear extinction.
Rosas-Vidal, LE; Do-Monte, FH; Sotres-Bayon, F; Quirk, GJ
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology
39
2161-9
2014
Show Abstract
Infusing brain-derived neurotrophic factor (BDNF) into the infralimbic (IL) prefrontal cortex is capable of inducing extinction. Little is known, however, about the circuits mediating BDNF effects on extinction or the extent to which extinction requires BDNF in IL. Using local pharmacological infusion of BDNF protein, or an antibody against BDNF, we found that BDNF in the IL, but not prelimbic (PL) prefrontal cortex, is both necessary and sufficient for fear extinction. Furthermore, we report that BDNF in IL can induce extinction of older fear memories (14 days) as well as recent fear memories (1 day). Using immunocytochemistry, we show that BDNF is increased in the ventral hippocampus (vHPC), but not IL or PL, following extinction training. Finally, we observed that infusing BDNF into the vHPC increased the firing rate of IL, but not PL neurons in fear conditioned rats. These findings indicate that an extinction-induced increase in BDNF within the vHPC enhances excitability in IL targets, thereby supporting extinction memories. | 24625752
 |
Feedback mechanism in depolarization-induced sustained activation of extracellular signal-regulated kinase in the hippocampus.
Maharana, C; Sharma, KP; Sharma, SK
Scientific reports
3
1103
2013
Show Abstract
Phosphorylation plays important roles in several processes including synaptic plasticity and memory. The critical role of extracellular signal-regulated kinase (ERK) in these processes is well established. ERK is activated in a sustained manner by different stimuli. However, the mechanisms of sustained ERK activation are not completely understood. Here we show that KCl depolarization-induced sustained ERK activation in the hippocampal slices is critically dependent on protein synthesis and transcription. In addition, the sustained ERK activation requires receptor tyrosine kinase(s) activity. In support of a role for a growth factor in sustained ERK activation, KCl depolarization enhances the level of brain-derived neurotrophic factor (BDNF). Furthermore, BDNF antibody blocks KCl-induced sustained ERK activation. These results suggest a positive feed-back loop in which depolarization-induced BDNF maintains ERK activation in the sustained phase. | 23346360
 |
Trk activation of the ERK1/2 kinase pathway stimulates intermediate chain phosphorylation and recruits cytoplasmic dynein to signaling endosomes for retrograde axonal transport.
Mitchell, DJ; Blasier, KR; Jeffery, ED; Ross, MW; Pullikuth, AK; Suo, D; Park, J; Smiley, WR; Lo, KW; Shabanowitz, J; Deppmann, CD; Trinidad, JC; Hunt, DF; Catling, AD; Pfister, KK
The Journal of neuroscience : the official journal of the Society for Neuroscience
32
15495-510
2012
Show Abstract
The retrograde transport of Trk-containing endosomes from the axon to the cell body by cytoplasmic dynein is necessary for axonal and neuronal survival. We investigated the recruitment of dynein to signaling endosomes in rat embryonic neurons and PC12 cells. We identified a novel phosphoserine on the dynein intermediate chains (ICs), and we observed a time-dependent neurotrophin-stimulated increase in intermediate chain phosphorylation on this site in both cell types. Pharmacological studies, overexpression of constitutively active MAP kinase kinase, and an in vitro assay with recombinant proteins demonstrated that the intermediate chains are phosphorylated by the MAP kinase ERK1/2, extracellular signal-regulated kinase, a major downstream effector of Trk. Live cell imaging with fluorescently tagged IC mutants demonstrated that the dephosphomimic mutants had significantly reduced colocalization with Trk and Rab7, but not a mitochondrial marker. The phosphorylated intermediate chains were enriched on immunoaffinity-purified Trk-containing organelles. Inhibition of ERK reduced the amount of phospho-IC and the total amount of dynein that copurified with the signaling endosomes. In addition, inhibition of ERK1/2 reduced the motility of Rab7- and TrkB-containing endosomes and the extent of their colocalization with dynein in axons. NGF-dependent survival of sympathetic neurons was significantly reduced by the overexpression of the dephosphomimic mutant IC-1B-S80A, but not WT IC-1B, further demonstrating the functional significance of phosphorylation on this site. These results demonstrate that neurotrophin binding to Trk initiates the recruitment of cytoplasmic dynein to signaling endosomes through ERK1/2 phosphorylation of intermediate chains for their subsequent retrograde transport in axons. | 23115187
 |
Motoneuron programmed cell death in response to proBDNF.
Taylor, AR; Gifondorwa, DJ; Robinson, MB; Strupe, JL; Prevette, D; Johnson, JE; Hempstead, B; Oppenheim, RW; Milligan, CE
Developmental neurobiology
72
699-712
2012
Show Abstract
Motoneurons (MN) as well as most neuronal populations undergo a temporally and spatially specific period of programmed cell death (PCD). Several factors have been considered to regulate the survival of MNs during this period, including availability of muscle-derived trophic support and activity. The possibility that target-derived factors may also negatively regulate MN survival has been considered, but not pursued. Neurotrophin precursors, through their interaction with p75(NTR) and sortilin receptors have been shown to induce cell death during development and following injury in the CNS. In this study, we find that muscle cells produce and secrete proBDNF. ProBDNF through its interaction with p75(NTR) and sortilin, promotes a caspase-dependent death of MNs in culture. We also provide data to suggest that proBDNF regulates MN PCD during development in vivo. | 21834083
 |
Smilagenin attenuates beta amyloid (25-35)-induced degeneration of neuronal cells via stimulating the gene expression of brain-derived neurotrophic factor.
R Zhang,Z Wang,P A Howson,Z Xia,S Zhou,E Wu,Y Hu
Neuroscience
210
2012
Show Abstract
The development of drugs that attenuate neurodegeneration is important for the treatment of Alzheimer's disease (AD). We previously found that smilagenin (SMI), a steroidal sapogenin from traditional Chinese medicinal herbs improves memory in animal models, is neither a cholinesterase inhibitor nor a glutamate receptor antagonist, but can significantly elevate the declined muscarinic receptor (M receptor) density. In this article, to clarify whether SMI represents a new approach for treating neurodegeneration disease, we first demonstrate that SMI pretreatment significantly attenuates the neurodegenerative changes induced by beta amyloid 25-35 (Aβ(25-35)) in cultured rat cortical neurons, including decreased cholinergic neuron number, shortened neurite outgrowth length, and declined M receptor density. Brain-derived neurotrophic factor (BDNF) protein levels in the culture medium were also decreased by Aβ(25-35) and significantly elevated by SMI. Parallel experiments revealed that when the trk receptors were inhibited by K252a or the action of BDNF was inhibited by a neutralizing anti-BDNF antibody, the effects of SMI on the Aβ(25-35)-induced neurodegeneration in rat cortical neurons were almost completely abolished. In the all-trans retinoic acid (RA)-differentiated SH-SY5Y neuroblastoma cells, the BDNF transcription rate measured by a nuclear run-on assay was significantly suppressed by Aβ(25-35) and elevated by SMI, but the BDNF degradation rate measured by half-life determination was unchanged by Aβ(25-35) and SMI. Transcript analysis of the SH-SY5Y cells using quantitative RT-PCR (qRT-PCR) showed that the IV and VI transcripts of BDNF mRNA were significantly decreased by Aβ(25-35) and elevated by SMI. Taken together, we conclude that SMI attenuates Aβ(25-35)-induced neurodegeneration in cultured rat cortical neurons and SH-SY5Y cells mainly through stimulating BDNF mRNA transcription implicating that SMI may represent a novel therapeutic strategy for AD. | 22441042
 |
Upregulation of CREB-mediated transcription enhances both short- and long-term memory.
Suzuki, A; Fukushima, H; Mukawa, T; Toyoda, H; Wu, LJ; Zhao, MG; Xu, H; Shang, Y; Endoh, K; Iwamoto, T; Mamiya, N; Okano, E; Hasegawa, S; Mercaldo, V; Zhang, Y; Maeda, R; Ohta, M; Josselyn, SA; Zhuo, M; Kida, S
The Journal of neuroscience : the official journal of the Society for Neuroscience
31
8786-802
2011
Show Abstract
Unraveling the mechanisms by which the molecular manipulation of genes of interest enhances cognitive function is important to establish genetic therapies for cognitive disorders. Although CREB is thought to positively regulate formation of long-term memory (LTM), gain-of-function effects of CREB remain poorly understood, especially at the behavioral level. To address this, we generated four lines of transgenic mice expressing dominant active CREB mutants (CREB-Y134F or CREB-DIEDML) in the forebrain that exhibited moderate upregulation of CREB activity. These transgenic lines improved not only LTM but also long-lasting long-term potentiation in the CA1 area in the hippocampus. However, we also observed enhanced short-term memory (STM) in contextual fear-conditioning and social recognition tasks. Enhanced LTM and STM could be dissociated behaviorally in these four lines of transgenic mice, suggesting that the underlying mechanism for enhanced STM and LTM are distinct. LTM enhancement seems to be attributable to the improvement of memory consolidation by the upregulation of CREB transcriptional activity, whereas higher basal levels of BDNF, a CREB target gene, predicted enhanced shorter-term memory. The importance of BDNF in STM was verified by microinfusing BDNF or BDNF inhibitors into the hippocampus of wild-type or transgenic mice. Additionally, increasing BDNF further enhanced LTM in one of the lines of transgenic mice that displayed a normal BDNF level but enhanced LTM, suggesting that upregulation of BDNF and CREB activity cooperatively enhances LTM formation. Our findings suggest that CREB positively regulates memory consolidation and affects memory performance by regulating BDNF expression. | 21677163
 |
PSA-NCAM-dependent GDNF signaling limits neurodegeneration and epileptogenesis in temporal lobe epilepsy.
Venceslas Duveau,Jean-Marc Fritschy
The European journal of neuroscience
32
2010
Show Abstract
Polysialylated neuronal cell adhesion molecule (PSA-NCAM), a polysialylated protein constitutively expressed in the hippocampus, is involved in neuronal growth, synaptic plasticity and neurotrophin signaling. In particular, PSA-NCAM mediates Ret-independent glial-derived neurotrophic factor (GDNF) signaling, leading to downstream FAK activation. GDNF has potent seizure-suppressant action, whereas PSA-NCAM is upregulated by seizure activity. However, the involvement of Ret-independent GDNF signaling in temporal lobe epilepsy (TLE) is not established. We tested the effects of PSA-NCAM inactivation on neurodegeneration and epileptogenesis in a mouse model of TLE. In this model, unilateral intrahippocampal kainic acid (KA) injection induced degeneration of CA1, CA3c and hilar neurons, followed by spontaneous recurrent focal seizures. In the contralateral, morphologically preserved hippocampus, a long-lasting increase of PSA-NCAM immunoreactivity was observed. Inactivation of PSA-NCAM by endoneuraminidase (EndoN) administration into the contralateral ventricle of KA-treated mice caused severe degeneration of CA3a,b neurons and dentate gyrus granule cells in the epileptic focus, and led to early onset of focal seizures. This striking trans-hemispheric alteration suggested that PSA-NCAM mediates GDNF signaling, leading to transport of neuroprotective signals into the lesioned hippocampus. This hypothesis was confirmed by injecting GDNF antibodies into the contralateral hippocampus of KA-treated mice, thereby reproducing the enhanced neurodegeneration seen after PSA-NCAM inactivation. Furthermore, contralateral EndoN and anti-GDNF treatment decreased GDNF family receptor alpha1 immunoreactivity and FAK phosphorylation in the epileptic focus. Thus, Ret-independent GDNF signaling across the commissural projection might protect CA3a,b neurons and delay seizure onset. These findings implicate GDNF in the control of epileptogenesis and offer a possible mechanism explaining lesion asymmetry in mesial TLE. | 20597970
 |
Spinal alpha 2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function.
Ken-ichiro Hayashida,James C Eisenach
Anesthesiology
113
2010
Show Abstract
Spinal alpha2-adrenoceptor stimulation produces analgesia in neuropathic pain states, and this effect in animals is blocked by the inhibitors of brain-derived neurotrophic factor (BDNF) function. In rats, alpha2-adrenoceptor stimulation normally inhibits acetylcholine release, but it excites release after nerve injury. The authors examined the roles of BDNF and excitatory Gs-protein in this change. Full Text Article | 20613480
 |
Endogenous BDNF regulates induction of intrinsic neuronal growth programs in injured sensory neurons.
Nicole M Geremia,Lina M E Pettersson,J C Hasmatali,Todd Hryciw,Nils Danielsen,David J Schreyer,Valerie M K Verge
Experimental neurology
223
2010
Show Abstract
Identification of the molecule(s) that globally induce a robust regenerative state in sensory neurons following peripheral nerve injury remains elusive. A potential candidate is brain-derived neurotrophic factor (BDNF), the sole neurotrophin upregulated in sensory neurons after peripheral nerve injury. Here we tested the hypothesis that BDNF plays a critical role in the regenerative response of mature rat sensory neurons following peripheral nerve lesion. Neutralization of endogenous BDNF was performed by infusing BDNF antibodies intrathecally via a mini-osmotic pump for 3 days at the level of the fifth lumbar dorsal root ganglion, immediately following unilateral spinal nerve injury. This resulted in decreased expression of the injury/regeneration-associated genes growth-associated protein-43 and Talpha1 tubulin in the injured sensory neurons as compared to injury plus control IgG infused or injury alone animals. Similar results were observed following inhibition of BDNF expression by intrathecal delivery of small interfering RNAs (siRNA) targeting BDNF starting 3 days prior to injury. The reduced injury/regeneration-associated gene expression correlated with a significantly reduced intrinsic capacity of these neurons to extend neurites when assayed in vitro. In contrast, delayed infusion of BDNF antibody for 3 days beginning 1 week post-lesion had no discernible influence on the elevated expression of these regeneration-associated markers. These results support an important role for endogenous BDNF in induction of the cell body response in injured sensory neurons and their intrinsic ability to extend neurites, but BDNF does not appear to be necessary for maintaining the response once it is induced. | 19646438
 |