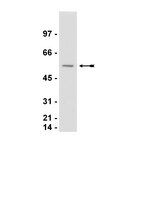
Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion.
Coppolino, M G, et al.
Nature, 386: 843-7 (1997)
1997
Show Abstract
Integrins are important mediators of cell adhesion to extracellular ligands and can transduce biochemical signals both into and out of cells. The cytoplasmic domains of integrins interact with several structural and signalling proteins and consequently participate in the regulation of cell shape, motility, growth and differentiation. It has been shown that calreticulin associates with the cytoplasmic domains of integrin alpha-subunits and that this interaction can influence integrin-mediated cell adhesion to extracellular matrix. We have now developed calreticulin-deficient embryonic stem (ES) cells and isolated embryonic fibroblasts from calreticulin mutant mice. We find that in both cell types integrin-mediated adhesion is severely impaired, although integrin expression is unaltered. Expression of recombinant calreticulin in double knockout ES cells by complementary DNA transfection rescued integrin-mediated adhesion. In wild-type cells, engagement of surface integrins induced a transient elevation in cytosolic calcium concentration owing to influx of extracellular calcium. This calcium transient was absent in calreticulin-deficient cells. In contrast, the amount of calcium in endomembrane stores, which is sensitive to both inositol 1,4,5-trisphosphate and thapsigargin, was indistinguishable in the two cell types. Our results indicate that calreticulin is an essential modulator both of integrin adhesive functions and integrin-initiated signalling, but that it may not play a significant role in the storage of luminal calcium. | 9126744
 |
Calreticulin inhibits glucocorticoid- but not cAMP-sensitive expression of tyrosine aminotransferase gene in cultured McA-RH7777 hepatocytes.
Burns, K, et al.
Mol. Cell. Biochem., 171: 37-43 (1997)
1997
Show Abstract
Calreticulin is a ubiquitously expressed Ca2+ binding protein of the endoplasmic reticulum which inhibits DNA binding and transcriptional activation by steroid hormone receptors. In this study the effects of calreticulin on tyrosine aminotransferase (TAT) gene expression in cultured McA-RH7777 hepatocytes was investigated. McA-RH7777 cells were stably transfected with calreticulin expression vector to generate cells overexpressing the protein. The transcriptional activity of the TAT gene, which is glucocorticoid-sensitive and cAMP-dependent, was investigated in the mock transfected McA-RH7777 and in cells overexpressing calreticulin (designated McA-11 and McA-17). In the presence of dexamethasone or the cAMP analog (CTP-cAMP) expression of the TAT gene was induced in mock transfected McA-RH7777 cells by approximately 4.5 and 5 fold, respectively. In McA-11 and McA-17 cells, overexpressing calreticulin, glucocorticoid-sensitive expression of the TAT gene was significantly inhibited, however, the CTP-cAMP-dependent expression of the TAT gene was not affected. The ability of calreticulin to inhibit glucocorticoid-sensitive TAT gene expression but not the cAMP-dependent expression of the gene suggests that the protein affects specifically the action of transcription pathways involving steroid receptors or transcription factors containing KxFF(K/R)R-like motifs. Calreticulin may play an important role in the regulation of glucocorticoid-sensitive pathway of expression of the hepatocytes specific genes during development. | 9201693
 |
Regulation of calreticulin gene expression by calcium.
Waser, M, et al.
J. Cell Biol., 138: 547-57 (1997)
1997
Show Abstract
We have isolated and characterized a 12-kb mouse genomic DNA fragment containing the entire calreticulin gene and 2.14 kb of the promoter region. The mouse calreticulin gene consists of nine exons and eight introns, and it spans 4.2 kb of genomic DNA. A 1.8-kb fragment of the calreticulin promoter was subcloned into a reporter gene plasmid containing chloramphenicol acetyltransferase. This construct was then used in transient and stable transfection of NIH/ 3T3 cells. Treatment of transfected cells either with the Ca2+ ionophore A23187, or with the ER Ca2+-ATPase inhibitor thapsigargin, resulted in a five- to sevenfold increase of the expression of chloramphenicol acetyltransferase protein. Transactivation of the calreticulin promoter was also increased by fourfold in NIH/3T3 cells treated with bradykinin, a hormone that induces Ca2+ release from the intracellular Ca2+ stores. Analysis of the promoter deletion constructs revealed that A23187- and thapsigargin-responsive regions are confined to two regions (-115 to -260 and -685 to -1,763) in the calreticulin promoter that contain the CCAAT nucleotide sequences. Northern blot analysis of cells treated with A23187, or with thapsigargin, revealed a fivefold increase in calreticulin mRNA levels. Thapsigargin also induced a fourfold increase in calreticulun protein levels. Importantly, we show by nuclear run-on transcription analysis that calreticulin gene transcription is increased in NIH/3T3 cells treated with A23187 and thapsigargin in vivo. This increase in gene expression required over 4 h of continuous incubation with the drugs and was also sensitive to treatment with cycloheximide, suggesting that it is dependent on protein synthesis. Changes in the concentration of extracellular and cytoplasmic Ca2+ did not affect the increased expression of the calreticulin gene. These studies suggest that stress response to the depletion of intracellular Ca2+ stores induces expression of the calreticulin gene in vitro and in vivo. | 9245785
 |
Endoplasmic reticulum form of calreticulin modulates glucocorticoid-sensitive gene expression.
Michalak, M, et al.
J. Biol. Chem., 271: 29436-45 (1996)
1996
Show Abstract
Calreticulin is a ubiquitously expressed Ca2+-binding protein of the endoplasmic reticulum (ER), which inhibits DNA binding in vitro and transcriptional activation in vivo by steroid hormone receptors. Transient transfection assays were carried out to investigate the effects of different intracellular targeting of calreticulin on transactivation mediated by glucocorticoid receptor. BSC40 cells were transfected with either calreticulin expression vector (ER form of calreticulin) or calreticulin expression vector encoding calreticulin minus leader peptide, resulting in cytoplasmic localization of the recombinant protein. Transfection of BSC40 cells with calreticulin expression vector encoding the ER form of the protein led to 40-50% inhibition of the dexamethasone-sensitive stimulation of luciferase expression. However, in a similar experiment, but using the calreticulin expression vector encoding cytoplasmic calreticulin, dexamethasone-stimulated activation of the luciferase reporter gene was inhibited by only 10%. We conclude that the ER, but not cytosolic, form of calreticulin is responsible for inhibition of glucocorticoid receptor-mediated gene expression. These effects are specific to calreticulin, since overexpression of the ER lumenal proteins (BiP, ERp72, or calsequestrin) has no effect on glucocorticoid-sensitive gene expression. The N domain of calreticulin binds to the DNA binding domain of the glucocorticoid receptor in vitro; however, we show that the N+P domain of calreticulin, when synthesized without the ER signal sequence, does not inhibit glucocorticoid receptor function in vivo. Furthermore, expression of the N domain of calreticulin and the DNA binding domain of glucocorticoid receptor as fusion proteins with GAL4 in the yeast two-hybrid system revealed that calreticulin does not interact with glucocorticoid receptor under these conditions. We conclude that calreticulin and glucocorticoid receptor may not interact in vivo and that the calreticulin-dependent modulation of the glucocorticoid receptor function may therefore be due to a calreticulin-dependent signaling from the ER. | 8910610
 |
Calreticulin, and not calsequestrin, is the major calcium binding protein of smooth muscle sarcoplasmic reticulum and liver endoplasmic reticulum.
Milner, R E, et al.
J. Biol. Chem., 266: 7155-65 (1991)
1991
Show Abstract
The distribution of calsequestrin and calreticulin in smooth muscle and non-muscle tissues was investigated. Immunoblots of endoplasmic reticulum proteins probed with anti-calreticulin and anti-calsequestrin antibodies revealed that only calreticulin is present in the rat liver endoplasmic reticulum. Membrane fractions isolated from uterine smooth muscle, which are enriched in sarcoplasmic reticulum, contain a protein band which is immunoreactive with anti-calreticulin but not with anti-calsequestrin antibodies. The presence of calreticulin in these membrane fractions was further confirmed by 45Ca2+ overlay and "Stains-All" techniques. Calreticulin was also localized to smooth muscle sarcoplasmic reticulum by the indirect immunofluorescence staining of smooth muscle cells with anti-calreticulin antibodies. Furthermore, both liver and uterine smooth muscle were found to contain high levels of mRNA encoding calreticulin, whereas no mRNA encoding calsequestrin was detected. We have employed an ammonium sulfate precipitation followed by Mono Q fast protein liquid chromatography, as a method by which calsequestrin and calreticulin can be isolated from whole tissue homogenates, and by which they can be clearly resolved from one another, even where present in the same tissue. Calreticulin was isolated from rabbit and bovine liver, rabbit brain, rabbit and porcine uterus, and bovine pancreas and was identified by its amino-terminal amino acid sequence. Calsequestrin cannot be detected in preparations from whole liver tissue, and only very small amounts of calsequestrin are detectable in ammonium sulfate extracts of uterine smooth muscle. We conclude that calreticulin, and not calsequestrin, is a major Ca2+ binding protein in liver endoplasmic reticulum and in uterine smooth muscle sarcoplasmic reticulum. Calsequestrin and calreticulin may perform parallel functions in the lumen of the sarcoplasmic and endoplasmic reticulum. | 2016321
 |