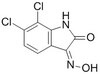
Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts.
Sonia Saad, David J Gottlieb, Kenneth F Bradstock, Christopher M Overall, Linda J Bendall
Cancer research
62
283-9
2002
Show Abstract
The bone and bone marrow are the most common sites of metastasis in breast cancer. Matrix metalloproteinases (MMPs), particularly MMP-2, produced by cancer cells or, more typically, induced in the adjacent normal stroma are necessary for the degradation of extracellular matrix essential for cancer metastasis. Here we describe a mechanism by which breast cancer cells can rapidly use MMP-2 produced by bone marrow fibroblasts (BMFs). MMP-2 is stored in an inactive conformation in association with the cell surface or extracellular matrix of BMFs. Cocultures of BMFs and the human breast cancer cell line MDA-MB-231 induce release of MMP-2 into the culture supernatant without up-regulation of MMP-2 synthesis in either cell. MMP-2 is present on the surface of BMFs and is displaced by MDA-MB-231 cells or by fibronectin or fragments of fibronectin containing the fibronectin type II modules. Moreover, when fibronectin is eluted from the surface of MDA-MB-231 cells, they lose the ability to induce the release of MMP-2 from BMFs. These data are consistent with the displacement of inactive MMP-2 bound to normal fibroblasts via its collagen-binding domain by fibronectin type II modules of cancer cell-associated fibronectin. Cancer cells can then use the proteinase to facilitate tissue invasion. Because an identical mechanism can be demonstrated using fibroblasts from different sources, it is likely to be important for the rapid movement of malignant cells into a variety of normal tissues. | 11782389
 |