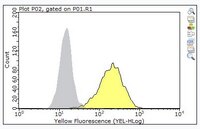
Bispecific monoclonal antibodies mediate binding of dengue virus to erythrocytes in a monkey model of passive viremia.
Hahn, CS; French, OG; Foley, P; Martin, EN; Taylor, RP
J Immunol
166
1057-65
2001
Show Abstract
Dengue viruses (DEN), causative agents of dengue fever (DF) and more severe dengue hemorrhagic fever (DHF)/dengue shock syndrome, infect over 100 million people every year. Among those infected, up to one-half million people develop DHF, which requires an extensive hospital stay. Recent reports indicate that there is a significant correlation between virus titer in the bloodstream of infected individuals and the severity of the disease, especially the development of DHF. This suggests that if there is a procedure to reduce viremia in infected subjects, then the severity of the disease may be controlled during the critical early stages of the disease before it progresses to DHF. We have generated bispecific mAb complexes (heteropolymer(s), HP), which contain a mAb specific for the DEN envelope glycoprotein cross-linked with a second mAb specific for the primate E complement receptor 1. These HP facilitate rapid binding of DEN to human and monkey E in vitro, with approximately 90% bound within 5 min. Furthermore, in a passive viremia monkey model established by continuous steady state infusion of DEN, injection of HP during the steady state promoted rapid binding of DEN to the E, followed by subsequent clearance from the vascular system. Moreover, HP previously infused into the circulation is capable of efficiently capturing a subsequent challenge dose of DEN and binding it to E. These data suggest that HP potentially can be useful for alleviating DEN infection-associated symptoms by reducing titers of free virus in the vascular system. | 11145685
 |
Mapping epitopes for 20 monoclonal antibodies to CR1.
Nickells, M; Hauhart, R; Krych, M; Subramanian, VB; Geoghegan-Barek, K; Marsh, HC; Atkinson, JP
Clin Exp Immunol
115
27-36
2001
Show Abstract
Complement receptor type one (CR1; CD35) binds and processes C3b and C4b opsonized immune complexes and regulates complement activation. We have characterized the epitopes of 13 previously reported and seven new MoAbs to human CR1. The MoAbs formed seven groups based on their reactivity with a panel of deletion forms of CR1. Seventeen of the MoAbs reacted with CR1 at more than one site, a consequence of its repetitive sequence. All five of the MoAbs recognizing epitopes in the nearly identical repeats 3, 10, and 17, as well as one MoAb which reacted with repeats 8 or 1/2 of 9 and 15 or 1/2 of 16, blocked cofactor activity for C3b. Knowledge of the repeats bearing the epitopes for these MoAbs should facilitate the further characterization of CR1. | 9566786
 |