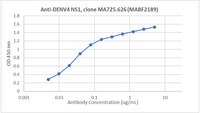
Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries.
Burritt, JB; Quinn, MT; Jutila, MA; Bond, CW; Jesaitis, AJ
J Biol Chem
270
16974-80
1995
Show Abstract
Cytochrome b of human neutrophils is the central component of the microbicidal NADPH-oxidase system. However, the folding topology of this integral membrane protein remains undetermined. Two random-sequence bacteriophage peptide libraries were used to map structural features of cytochrome b by determining the epitopes of monoclonal antibodies (mAbs) 44.1 and 54.1, specific for the p22phox and gp91phox cytochrome b chains, respectively. The unique peptides of phage selected by mAb affinity purification were deduced from the phage DNA sequences. Phage selected by mAb 44.1 displayed the consensus peptide sequence GGPQVXPI, which is nearly identical to 181GGPQVNPI18 of p22phox. Phage selected by mAb 54.1 displayed the consensus sequence PKXAVDGP, which resembles 382PKIAVDGP389 of gp91phox. Western blotting demonstrated specific binding of each mAb to the respective cytochrome b subunit and selected phage peptides. In flow cytometric analysis, mAb 44.1 bound only permeabilized neutrophils, while 54.1 did not bind intact or permeabilized cells. However, mAb 54.1 immunosedimented detergent-solubilized cytochrome b in sucrose gradients. These results suggest the 181GGPQVNPI188 segment of p22phox is accessible on its intracellular surface, but the 382PKIAVDGP389 region on gp91phox is not accessible to antibody, and probably not on the protein surface. | 7622517
 |