Role of SUMOylation in Full Antiestrogenicity.
Khalid Hilmi,Nader Hussein,Rodrigo Mendoza-Sanchez,Mohamed El-Ezzy,Houssam Ismail,Chantal Durette,Martine Bail,Maria Johanna Rozendaal,Michel Bouvier,Pierre Thibault,James L Gleason,Sylvie Mader
Molecular and cellular biology
32
2012
Show Abstract
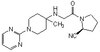
The selective estrogen receptor downregulator (SERD) fulvestrant can be used as second-line treatment for patients relapsing after treatment with tamoxifen, a selective estrogen receptor modulator (SERM). Unlike tamoxifen, SERDs are devoid of partial agonist activity. While the full antiestrogenicity of SERDs may result in part from their capacity to downregulate levels of estrogen receptor alpha (ERα) through proteasome-mediated degradation, SERDs are also fully antiestrogenic in the absence of increased receptor turnover in HepG2 cells. Here we report that SERDs induce the rapid and strong SUMOylation of ERα in ERα-positive and -negative cell lines, including HepG2 cells. Four sites of SUMOylation were identified by mass spectrometry analysis. In derivatives of the SERD ICI164,384, SUMOylation was dependent on the length of the side chain and correlated with full antiestrogenicity. Preventing SUMOylation by the overexpression of a SUMO-specific protease (SENP) deSUMOylase partially derepressed transcription in the presence of full antiestrogens in HepG2 cells without a corresponding increase in activity in the presence of agonists or of the SERM tamoxifen. Mutations increasing transcriptional activity in the presence of full antiestrogens reduced SUMOylation levels and suppressed stimulation by SENP1. Our results indicate that ERα SUMOylation contributes to full antiestrogenicity in the absence of accelerated receptor turnover. | 22826433
 |
Expression of estrogen receptor ? and ? in rat astrocytes in primary culture: effects of hypoxia and glucose deprivation.
M D Al-Bader,S A Malatiali,Z B Redzic
Physiological research / Academia Scientiarum Bohemoslovaca
60
2011
Show Abstract
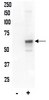
Estrogen replacement therapy could play a role in the reduction of injury associated with cerebral ischemia in vivo, which could be, at least partially, a consequence of estrogen influence of glutamate buffering by astrocytes during hypoxia/ischemia. Estrogen exerts biological effects through interaction with its two receptors: estrogen receptor alpha (ER?) and estrogen receptor beta (ER?), which are both expressed in astrocytes. This study explored effects of hypoxia and glucose deprivation (HGD), alone or followed by 1 h recovery, on ER? and ER? expression in primary rat astrocyte cultures following 1 h exposure to: a) 5 % CO(2) in air (control group-CG); b) 2 % O(2)/5 % CO(2) in N(2) with glucose deprivation (HGD group-HGDG); or c) the HGDG protocol followed by 1 h CG protocol (recovery group-RG). ER? mRNA expression decreased in HGDG. At the protein level, full-length ER? (67 kDa) and three ER?-immunoreactive protein bands (63, 60 and 52 kDa) were detected. A significant decrease in the 52 kDa band was seen in HGDG, while a significant decrease in expression of the full length ER? was seen in the RG. ER? mRNA and protein expression (a 54 kDa single band) did not change. The observed decrease in ER? protein may limit estrogen-mediated signalling in astrocytes during hypoxia and recovery. | 21995903
 |
Deficiency of chemokine receptor CCR1 causes osteopenia due to impaired functions of osteoclasts and osteoblasts.
Hoshino, A; Iimura, T; Ueha, S; Hanada, S; Maruoka, Y; Mayahara, M; Suzuki, K; Imai, T; Ito, M; Manome, Y; Yasuhara, M; Kirino, T; Yamaguchi, A; Matsushima, K; Yamamoto, K
The Journal of biological chemistry
285
28826-37
2010
Show Abstract
Chemokines are characterized by the homing activity of leukocytes to targeted inflammation sites. Recent research indicates that chemokines play more divergent roles in various phases of pathogenesis as well as immune reactions. The chemokine receptor, CCR1, and its ligands are thought to be involved in inflammatory bone destruction, but their physiological roles in the bone metabolism in vivo have not yet been elucidated. In the present study, we investigated the roles of CCR1 in bone metabolism using CCR1-deficient mice. Ccr1(-/-) mice have fewer and thinner trabecular bones and low mineral bone density in cancellous bones. The lack of CCR1 affects the differentiation and function of osteoblasts. Runx2, Atf4, Osteopontin, and Osteonectin were significantly up-regulated in Ccr1(-/-) mice despite sustained expression of Osterix and reduced expression of Osteocalcin, suggesting a lower potential for differentiation into mature osteoblasts. In addition, mineralized nodule formation was markedly disrupted in cultured osteoblastic cells isolated from Ccr1(-/-) mice. Osteoclastogenesis induced from cultured Ccr1(-/-) bone marrow cells yielded fewer and smaller osteoclasts due to the abrogated cell-fusion. Ccr1(-/-) osteoclasts exerted no osteolytic activity concomitant with reduced expressions of Rank and its downstream targets, implying that the defective osteoclastogenesis is involved in the bone phenotype in Ccr1(-/-) mice. The co-culture of wild-type osteoclast precursors with Ccr1(-/-) osteoblasts failed to facilitate osteoclastogenesis. This finding is most likely due to a reduction in Rankl expression. These observations suggest that the axis of CCR1 and its ligands are likely to be involved in cross-talk between osteoclasts and osteoblasts by modulating the RANK-RANKL-mediated interaction. | 20571024
 |
Transcriptional activation of DNA-dependent protein kinase catalytic subunit gene expression by oestrogen receptor-alpha.
Medunjanin S, Weinert S, Poitz D, Schmeisser A, Strasser RH, Braun-Dullaeus RC
EMBO Rep
11
208-13. Epub 2010 Jan 29.
2010
Show Abstract
The cellular response to DNA double-strand break (DSB) occurs through an integrated sensing and signalling network that maintains genomic stability. Oestrogen (E2), among its many functions, is known to have a positive effect on global genomic DNA repair; however, the mechanism by which it functions is unclear. A central enzyme involved in DNA DSB repair in mammalian cells is the DNA-dependent protein kinase (DNA-PK). Here, we show that E2 enhances DNA-PK catalytic subunit (DNA-PKcs) promoter activity with subsequent transcriptional and translational upregulation of DNA-PKcs in a breast cancer cell line. We identify two potential E2 receptor-alpha (ERalpha)-binding sites in a region upstream from the DNA-PKcs initiation site. By using small interfering RNA and the specific E2 receptor antagonist ICI 182,780, we demonstrate that ERalpha knockdown reduces E2-induced upregulation of DNA-PKcs expression and activity in breast carcinoma cells. E2-induced DNA-PK transactivation results in an increased ability of the cells to repair DNA DSB. This previously unknown mechanism of DNA-PK regulation sheds new light on tumour biology and reveals new possibilities for the prevention and therapy of E2-sensitive proliferative diseases. Full Text Article | 20111054
 |
Interaction of the double-strand break repair kinase DNA-PK and estrogen receptor-alpha.
Medunjanin, S; Weinert, S; Schmeisser, A; Mayer, D; Braun-Dullaeus, RC
Molecular biology of the cell
21
1620-8
2010
Show Abstract
Estrogens are suggested to play a role in the development and progression of proliferative diseases such as breast cancer. Like other steroid hormone receptors, the estrogen receptor-alpha (ERalpha) is a substrate of protein kinases, and phosphorylation has profound effects on its function and activity. Given the importance of DNA-dependent protein kinase (DNA-PK) for DNA repair, cell cycle progression, and survival, we hypothesized that it modulates ERalpha signaling. Here we show that, upon estrogen stimulation, DNA-PK forms a complex with ERalpha in a breast cancer cell line (MELN). DNA-PK phosphorylates ERalpha at Ser-118. Phosphorylation resulted in stabilization of ERalpha protein as inhibition of DNA-PK resulted in its proteasomal degradation. Activation of DNA-PK by double-strand breaks or its inhibition by siRNA technology demonstrated that estrogen-induced ERalpha activation and cell cycle progression is, at least, partially dependent on DNA-PK. Full Text Article | 20219974
 |
The poly(A)-dependent transcriptional pause is mediated by CPSF acting on the body of the polymerase.
Anita Nag,Kazim Narsinh,Harold G Martinson
Nature structural & molecular biology
14
2007
Show Abstract
Eukaryotic poly(A) signals direct mRNA 3'-end processing and also pausing and termination of transcription. We show that pausing and termination require the processing factor CPSF, which binds the AAUAAA hexamer of the mammalian poly(A) signal. Pausing does not require the RNA polymerase II C-terminal domain (CTD) or the cleavage stimulation factor, CstF, that binds the CTD. Pull-down experiments show that CPSF binds, principally through its 30-kDa subunit, to the body of the polymerase. CPSF can also bind CstF, but this seems to be mutually exclusive with polymerase binding. We suggest that CPSF, while binding the body of the polymerase, scans for hexamers in the extruding RNA. Any encounter with a hexamer triggers pausing. If the hexamer is part of a functional poly(A) signal, CstF is recruited and binds CPSF, causing it to release the polymerase body and move (with CstF) to the CTD. | 17572685
 |
Tumour necrosis factor and PI3-kinase control oestrogen receptor alpha protein level and its transrepression function.
Bhat-Nakshatri, P; Campbell, RA; Patel, NM; Newton, TR; King, AJ; Marshall, MS; Ali, S; Nakshatri, H
British journal of cancer
90
853-9
2004
Show Abstract
Oestrogen receptor alpha (ERalpha) is an oestrogen-activated transcription factor, which regulates proliferation and differentiation of mammary epithelial cells by activating or repressing gene expression. ERalpha is a critical prognostic indicator and a therapeutic target for breast cancer. Patients with tumours that express higher level of ERalpha have better prognosis than patients with tumours that are ERalpha negative or express lower level of ERalpha. Better prognosis in ERalpha-positive patients is believed to be due to repression of proinvasive gene expression by ERalpha. Oestrogen receptor alpha represses gene expression by transrepressing the activity of the transcription factors such as nuclear factor-kappaB or by inducing the expression of transcriptional suppressors such as MTA3. In this report, we show that ERalpha transrepresses the expression of the proinvasive gene interleukin 6 (IL-6) in ERalpha-negative MDA-MB-231 breast cancer cells stably overexpressing ERalpha. Using these cells as well as ERalpha-positive MCF-7 and ZR-75-1 cells, we show that tumour necrosis factor alpha (TNFalpha) and the phosphatidylinositol-3-kinase (PI3-kinase) modulate transrepression function of ERalpha by reducing its stability. From these results, we propose that TNFalpha expression or PI3-kinase activation lead to reduced levels of ERalpha protein in cancer cells and corresponding loss of transrepression function and acquisition of an invasive phenotype. | 14970864
 |
Loss of estrogen receptor signaling triggers epigenetic silencing of downstream targets in breast cancer.
Leu, YW; Yan, PS; Fan, M; Jin, VX; Liu, JC; Curran, EM; Welshons, WV; Wei, SH; Davuluri, RV; Plass, C; Nephew, KP; Huang, TH
Cancer research
64
8184-92
2004
Show Abstract
Alterations in histones, chromatin-related proteins, and DNA methylation contribute to transcriptional silencing in cancer, but the sequence of these molecular events is not well understood. Here we demonstrate that on disruption of estrogen receptor (ER) alpha signaling by small interfering RNA, polycomb repressors and histone deacetylases are recruited to initiate stable repression of the progesterone receptor (PR) gene, a known ERalpha target, in breast cancer cells. The event is accompanied by acquired DNA methylation of the PR promoter, leaving a stable mark that can be inherited by cancer cell progeny. Reestablishing ERalpha signaling alone was not sufficient to reactivate the PR gene; reactivation of the PR gene also requires DNA demethylation. Methylation microarray analysis further showed that progressive DNA methylation occurs in multiple ERalpha targets in breast cancer genomes. The results imply, for the first time, the significance of epigenetic regulation on ERalpha target genes, providing new direction for research in this classical signaling pathway. | 15548683
 |
EphA2 overexpression decreases estrogen dependence and tamoxifen sensitivity.
Ming Lu, Kathy D Miller, Yesim Gokmen-Polar, Meei-Huey Jeng, Michael S Kinch
Cancer research
63
3425-9
2003
Show Abstract
The EphA2 receptor tyrosine kinase is found at low levels on nontransformed adult breast epithelial cells but is frequently overexpressed on aggressive breast cancer cells. Recent studies have documented an inverse relationship between EphA2 and estrogen receptor expression in breast cancer cell lines. In our present study, we demonstrate that overexpression of EphA2 decreases estrogen dependence as defined using both in vitro and in vivo criteria. The EphA2-transfected cells demonstrate increased growth in vitro and form larger and more aggressive tumors in vivo. EphA2 overexpression also decreases the ability of tamoxifen to inhibit breast cancer cell growth and tumorigenesis. These effects of EphA2 overexpression can be overcome by antibody-based targeting of EphA2. In particular, certain EphA2 antibodies can resensitize EphA2-overexpressing breast tumor cells to tamoxifen. These results have important implications for understanding the molecular basis underlying estrogen dependence and provide further evidence that EphA2 may provide a much-needed therapeutic target for breast cancer. | 12810680
 |
Estrogen can prevent or reverse obesity and diabetes in mice expressing human islet amyloid polypeptide.
John G Geisler, Walter Zawalich, Kathleen Zawalich, Jonathan R T Lakey, Hans Stukenbrok, Anthony J Milici, Walter C Soeller
Diabetes
51
2158-69
2002
Show Abstract
Type 2 diabetes is characterized by loss of beta-cell mass and concomitant deposition of amyloid derived from islet amyloid polypeptide (IAPP). Previously we have shown that expression of human IAPP (huIAPP) in islets of transgenic mice results in either a rapid onset of hyperglycemia in mice homozygous for the huIAPP transgene on a lean background (FVB/N) or a gradual hyperglycemia in mice hemizygous for the huIAPP transgene on an obese background (A(vy)/A). In both strains, only the males routinely develop diabetes. To investigate this sexual dimorphism, we treated young prediabetic A(vy)/A mice transgenic for huIAPP (huIAPP-A(vy)) with 17beta-estradiol (E2). The treatment completely blocked the progression to hyperglycemia but also prevented the associated weight gain in these mice. Immunohistochemistry of pancreatic sections demonstrated normal islet morphology with no apparent deposition of islet amyloid. E2 treatment of 1-year-old huIAPP-A(vy) diabetic males rapidly reverses obesity and hyperglycemia. To determine the effects of E2 in a nonobese model, we also treated prediabetic, ad libitum-fed and pair-fed Lean-huIAPP transgenic males. E2 completely blocked the progression to hyperglycemia with no significant effect on body weight. Pancreatic insulin content and plasma insulin concentration of Lean-huIAPP transgenic mice increased in a dose-dependent manner. We demonstrated the presence of estrogen receptor (ER)-alpha mRNA in mouse and human islets. By also confirming the presence of ER-alpha protein in islets, we discovered a novel 58-kDa ER-alpha isoform in mice and a 52-kDa isoform in humans, in the absence of the classic 67-kDa protein found in most tissues of both species. The demonstrated presence of ER-alpha in mouse and human islets is consistent with a direct effect on islet function. We conclude that exogenous E2 administered to male mice may block human IAPP-mediated beta-cell loss both by direct action on beta-cells and by decreasing insulin demand through inhibition of weight gain or increasing insulin action. | 12086946
 |