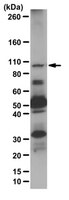
The Flavone Luteolin Suppresses SREBP-2 Expression and Post-Translational Activation in Hepatic Cells.
Wong, TY; Lin, SM; Leung, LK
PloS one
10
e0135637
2015
Show Abstract
High blood cholesterol has been associated with cardiovascular diseases. The enzyme HMG CoA reductase (HMGCR) is responsible for cholesterol synthesis, and inhibitors of this enzyme (statins) have been used clinically to control blood cholesterol. Sterol regulatory element binding protein (SREBP) -2 is a key transcription factor in cholesterol metabolism, and HMGCR is a target gene of SREBP-2. Attenuating SREBP-2 activity could potentially minimize the expression of HMGCR. Luteolin is a flavone that is commonly detected in plant foods. In the present study, Luteolin suppressed the expression of SREBP-2 at concentrations as low as 1 μM in the hepatic cell lines WRL and HepG2. This flavone also prevented the nuclear translocation of SREBP-2. Post-translational processing of SREBP-2 protein was required for nuclear translocation. Luteolin partially blocked this activation route through increased AMP kinase (AMPK) activation. At the transcriptional level, the mRNA and protein expression of SREBP-2 were reduced through luteolin. A reporter gene assay also verified that the transcription of SREBF2 was weakened in response to this flavone. The reduced expression and protein processing of SREBP-2 resulted in decreased nuclear translocation. Thus, the transcription of HMGCR was also decreased after luteolin treatment. In summary, the results of the present study showed that luteolin modulates HMGCR transcription by decreasing the expression and nuclear translocation of SREBP-2. | 26302339
 |
eIF6 coordinates insulin sensitivity and lipid metabolism by coupling translation to transcription.
Brina, D; Miluzio, A; Ricciardi, S; Clarke, K; Davidsen, PK; Viero, G; Tebaldi, T; Offenhäuser, N; Rozman, J; Rathkolb, B; Neschen, S; Klingenspor, M; Wolf, E; Gailus-Durner, V; Fuchs, H; Hrabe de Angelis, M; Quattrone, A; Falciani, F; Biffo, S
Nature communications
6
8261
2015
Show Abstract
Insulin regulates glycaemia, lipogenesis and increases mRNA translation. Cells with reduced eukaryotic initiation factor 6 (eIF6) do not increase translation in response to insulin. The role of insulin-regulated translation is unknown. Here we show that reduction of insulin-regulated translation in mice heterozygous for eIF6 results in normal glycaemia, but less blood cholesterol and triglycerides. eIF6 controls fatty acid synthesis and glycolysis in a cell autonomous fashion. eIF6 acts by exerting translational control of adipogenic transcription factors like C/EBPβ, C/EBPδ and ATF4 that have G/C rich or uORF sequences in their 5' UTR. The outcome of the translational activation by eIF6 is a reshaping of gene expression with increased levels of lipogenic and glycolytic enzymes. Finally, eIF6 levels modulate histone acetylation and amounts of rate-limiting fatty acid synthase (Fasn) mRNA. Since obesity, type 2 diabetes, and cancer require a Fasn-driven lipogenic state, we propose that eIF6 could be a therapeutic target for these diseases. | 26383020
 |
Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer.
Zhang, Z; Hou, X; Shao, C; Li, J; Cheng, JX; Kuang, S; Ahmad, N; Ratliff, T; Liu, X
Cancer research
74
6635-47
2014
Show Abstract
Prostate cancer is thought to be driven by oxidative stress, lipid metabolism, androgen receptor (AR) signaling, and activation of the PI3K-AKT-mTOR pathway, but it is uncertain how they may become coordinated during progression to castration-resistant disease that remains incurable. The mitotic kinase polo-like kinase 1 (Plk1) is elevated in prostate cancer, where its expression is linked to tumor grade. Notably, Plk1 signaling and lipid metabolism were identified recently as two of the top five most upregulated pathways in a mouse xenograft model of human prostate cancer. Herein, we show that oxidative stress activates both the PI3K-AKT-mTOR pathway and AR signaling in a Plk1-dependent manner in prostate cells. Inhibition of the PI3K-AKT-mTOR pathway prevented oxidative stress-induced activation of AR signaling. Plk1 modulation also affected cholesteryl ester accumulation in prostate cancer via the SREBP pathway. Finally, Plk1 inhibition enhanced cellular responses to androgen signaling inhibitors (ASI) and overcame ASI resistance in both cultured prostate cancer cells and patient-derived tumor xenografts. Given that activation of AR signaling and the PI3K-AKT-mTOR pathway is sufficient to elevate SREBP-dependent expression of key lipid biosynthesis enzymes in castration-resistant prostate cancer (CRPC), our findings argued that Plk1 activation was responsible for coordinating and driving these processes to promote and sustain the development of this advanced stage of disease. Overall, our results offer a strong mechanistic rationale to evaluate Plk1 inhibitors in combination drug trials to enhance the efficacy of ASIs in CRPC. | 25252916
 |
Hidden disease susceptibility and sexual dimorphism in the heterozygous knockout of Cyp51 from cholesterol synthesis.
Lewinska, M; Juvan, P; Perse, M; Jeruc, J; Kos, S; Lorbek, G; Urlep, Z; Keber, R; Horvat, S; Rozman, D
PloS one
9
e112787
2014
Show Abstract
We examined the genotype-phenotype interactions of Cyp51+/- mice carrying one functional allele of lanosterol 14α-demethylase from cholesterol biosynthesis. No distinct developmental or morphological abnormalities were observed by routine visual inspection of Cyp51+/- and Cyp51+/+ mice and fertility was similar. We further collected a large data-set from female and male Cyp51+/- mice and controls fed for 16 weeks with three diets and applied linear regression modeling. We used 3 predictor variables (genotype, sex, diet), and 39 response variables corresponding to the organ characteristics (7), plasma parameters (7), and hepatic gene expression (25). We observed significant differences between Cyp51+/- and wild-type mice in organ characteristics and blood lipid profile. Hepatomegaly was observed in Cyp51+/- males, together with elevated total and low-density lipoprotein cholesterol. Cyp51+/- females fed high-fat, high-cholesterol diet were leaner and had elevated plasma corticosterone compared to controls. We observed elevated hepatocyte apoptosis, mitosis and lipid infiltration in heterozygous knockouts of both sexes. The Cyp51+/- females had a modified lipid storage homeostasis protecting them from weight-gain when fed high-fat high-cholesterol diet. Malfunction of one Cyp51 allele therefore initiates disease pathways towards cholesterol-linked liver pathologies and sex-dependent response to dietary challenge. | 25393872
 |