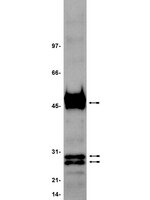
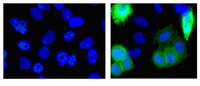
PRAME is a golgi-targeted protein that associates with the Elongin BC complex and is upregulated by interferon-gamma and bacterial PAMPs.
Wadelin, FR; Fulton, J; Collins, HM; Tertipis, N; Bottley, A; Spriggs, KA; Falcone, FH; Heery, DM
PloS one
8
e58052
2013
Show Abstract
Preferentially expressed antigen in melanoma (PRAME) has been described as a cancer-testis antigen and is associated with leukaemias and solid tumours. Here we show that PRAME gene transcription in leukaemic cell lines is rapidly induced by exposure of cells to bacterial PAMPs (pathogen associated molecular patterns) in combination with type 2 interferon (IFNγ). Treatment of HL60 cells with lipopolysaccharide or peptidoglycan in combination with IFNγ resulted in a rapid and transient induction of PRAME transcription, and increased association of PRAME transcripts with polysomes. Moreover, treatment with PAMPs/IFNγ also modulated the subcellular localisation of PRAME proteins in HL60 and U937 cells, resulting in targeting of cytoplasmic PRAME to the Golgi. Affinity purification studies revealed that PRAME associates with Elongin B and Elongin C, components of Cullin E3 ubiquitin ligase complexes. This occurs via direct interaction of PRAME with Elongin C, and PRAME colocalises with Elongins in the Golgi after PAMP/IFNγ treatment. PRAME was also found to co-immunoprecipitate core histones, consistent with its partial localisation to the nucleus, and was found to bind directly to histone H3 in vitro. Thus, PRAME is upregulated by signalling pathways that are activated in response to infection/inflammation, and its product may have dual functions as a histone-binding protein, and in directing ubiquitylation of target proteins for processing in the Golgi. | Western Blotting | | 23460923
 |
Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier.
Buffington, SA; Rasband, MN
The Journal of neuroscience : the official journal of the Society for Neuroscience
33
6191-202
2013
Show Abstract
The axon initial segment (AIS) and nodes of Ranvier are the sites of action potential initiation and regeneration in axons. Although the basic molecular architectures of AIS and nodes, characterized by dense clusters of Na(+) and K(+) channels, are similar, firing patterns vary among cell types. Neuronal firing patterns are established by the collective activity of voltage-gated ion channels and can be modulated through interaction with auxiliary subunits. Here, we report the neuronal expression pattern and subcellular localization of Navβ4, the modulatory Na(+) channel subunit thought to underlie resurgent Na(+) current. Immunostaining of rat tissues revealed that Navβ4 is strongly enriched at the AIS of a select set of neuron types, including many characterized by high-frequency firing, and at nodes of Ranvier in the PNS and some nodes in the CNS. By introducing full-length and mutant GFP-tagged Navβ4 into cultured neurons, we determined that the AIS and nodal localization of Navβ4 depends on its direct interaction with Na(+) channel α subunits through an extracellular disulfide bond. Based on these results, we propose that differences in the specific composition of the Na(+) channel complexes enriched at the AIS and nodes contribute to the diverse physiologies observed among cell types. | | Rat | 23554500
 |
Thrombospondin-related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes.
Robson, K J, et al.
EMBO J., 14: 3883-94 (1995)
1995
Show Abstract
Plasmodium sporozoites collected from oocysts, haemocoel and salivary glands of the mosquito show profound differences in their biological properties such as motility, ability to induce protective immune response and infectivity for vertebrate host cells. Sporozoites from salivary glands are much more infectious than those from oocysts and haemocoel. Differential expression of proteins, such as the circumsporozoite (CS) protein and the thrombospondin-related adhesive protein (TRAP), implicated in sporozoite recognition and entry into hepatocytes may account for the development of infectivity during ontogeny. We have carried out a series of experiments to: (i) analyse the expression and localization of TRAP in P.falciparum sporozoites during development in the mosquito; and (ii) elucidate the biochemical and adhesive properties of recombinant TRAP. Our data indicate that TRAP is not expressed in oocysts, whereas variable amounts of CS protein are found in this parasite developmental stage. Hemocoel sporozoites display the distinct phenotypes TRAP- CS protein+ and TRAP+ CS protein+ at a frequency of 98.5 and 1.5% respectively. Salivary gland sporozoites are all TRAP+ CS protein+. We also provide experimental evidence showing that recombinant TRAP binds to the basolateral cell membrane of hepatocytes in the Disse's space and that sulfated glycoconjugates function as TRAP ligands on human hepatocytes. | | | 7664729
 |