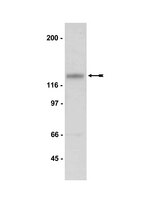
Gestational methylazoxymethanol exposure leads to NMDAR dysfunction in hippocampus during early development and lasting deficits in learning.
Snyder, MA; Adelman, AE; Gao, WJ
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology
38
328-40
2013
Show Abstract
The N-methyl-D-aspartate (NMDA) receptor has long been associated with learning and memory processes as well as diseased states, particularly in schizophrenia (SZ). Additionally, SZ is increasingly recognized as a neurodevelopmental disorder with cognitive impairments often preceding the onset of psychosis. However, the cause of these cognitive deficits and what initiates the pathological process is unknown. Growing evidence has implicated the glutamate system and, in particular, N-methyl-D-aspartate receptor (NMDAR) dysfunction in the pathophysiology of SZ. Yet, the vast majority of SZ-related research has focused on NMDAR function in adults leaving the role of NMDARs during development uncharacterized. We used the prenatal methylazoxymethanol acetate (MAM, E17) exposure model to determine the alterations of NMDAR protein levels and function, as well as associated cognitive deficits during development. We found that MAM-exposed animals have significantly altered NMDAR protein levels and function in the juvenile and adolescent hippocampus. Furthermore, these changes are associated with learning and memory deficits in the Morris Water Maze. Thus, in the prenatal MAM-exposure SZ model, NMDAR expression and function is altered during the critical period of hippocampal development. These changes may be involved in disease initiation and cognitive impairment in the early stage of SZ. | Western Blotting | 22968815
 |
The GluN3A subunit exerts a neuroprotective effect in brain ischemia and the hypoxia process.
Wang, H; Yan, H; Zhang, S; Wei, X; Zheng, J; Li, J
ASN neuro
5
231-42
2013
Show Abstract
NMDARs (N-methyl-D-aspartate receptors) mediate the predominantly excitatory neurotransmission in the CNS (central nervous system). Excessive release of glutamate and overactivation of NMDARs during brain ischemia and the hypoxia process are causally linked to excitotoxicity and neuronal damage. GluN3 subunits, the third member of the NMDAR family with two isoforms, GluN3A and GluN3B, have been confirmed to display an inhibitory effect on NMDAR activity. However, the effect of GluN3 subunits in brain ischemia and hypoxia is not clearly understood. In the present study, the influence of ischemia and hypoxia on GluN3 subunit expression was observed by using the 2VO (two-vessel occlusion) rat brain ischemia model and cell OGD (oxygen and glucose deprivation) hypoxia model. It was found that GluN3A protein expression in rat hippocampus and the prefrontal cortex was increased quickly after brain ischemia and remained at a high level for at least 24 h. However, the expression of the GluN3B subunit was not remarkably changed in both the animal and cell models. After OGD exposure, rat hippocampal neurons with GluN3A subunit overexpression displayed more viability than the wild-type neurons. NG108-15 cells overexpressing GluN3A presented pronounced resistance to glutamate insult. Blocking the increase of intracellular Ca2+ concentration may underlie the neuroprotective mechanism of up-regulated GluN3A subunit. Suppressing the generation of hydroxyl radicals and NO (nitric oxide) is probably also involved in the neuroprotection. | | 23883441
 |
Genetic deletion of NR3A accelerates glutamatergic synapse maturation.
Henson, MA; Larsen, RS; Lawson, SN; Pérez-Otaño, I; Nakanishi, N; Lipton, SA; Philpot, BD
PloS one
7
e42327
2012
Show Abstract
Glutamatergic synapse maturation is critically dependent upon activation of NMDA-type glutamate receptors (NMDARs); however, the contributions of NR3A subunit-containing NMDARs to this process have only begun to be considered. Here we characterized the expression of NR3A in the developing mouse forebrain and examined the consequences of NR3A deletion on excitatory synapse maturation. We found that NR3A is expressed in many subcellular compartments, and during early development, NR3A subunits are particularly concentrated in the postsynaptic density (PSD). NR3A levels dramatically decline with age and are no longer enriched at PSDs in juveniles and adults. Genetic deletion of NR3A accelerates glutamatergic synaptic transmission, as measured by AMPAR-mediated postsynaptic currents recorded in hippocampal CA1. Consistent with the functional observations, we observed that the deletion of NR3A accelerated the expression of the glutamate receptor subunits NR1, NR2A, and GluR1 in the PSD in postnatal day (P) 8 mice. These data support the idea that glutamate receptors concentrate at synapses earlier in NR3A-knockout (NR3A-KO) mice. The precocious maturation of both AMPAR function and glutamate receptor expression are transient in NR3A-KO mice, as AMPAR currents and glutamate receptor protein levels are similar in NR3A-KO and wildtype mice by P16, an age when endogenous NR3A levels are normally declining. Taken together, our data support a model whereby NR3A negatively regulates the developmental stabilization of glutamate receptors involved in excitatory neurotransmission, synaptogenesis, and spine growth. | Western Blotting | 22870318
 |
N-methyl-D-aspartate receptor subunit NR3a expression and function in principal cells of the collecting duct.
Sproul, A; Steele, SL; Thai, TL; Yu, S; Klein, JD; Sands, JM; Bell, PD
American journal of physiology. Renal physiology
301
F44-54
2011
Show Abstract
N-methyl-D-aspartate receptors (NMDARs) are Ca(2+)-permeable, ligand-gated, nonselective cation channels that function as neuronal synaptic receptors but which are also expressed in multiple peripheral tissues. Here, we show for the first time that NMDAR subunits NR3a and NR3b are highly expressed in the neonatal kidney and that there is continued expression of NR3a in the renal medulla and papilla of the adult mouse. NR3a was also expressed in mIMCD-3 cells, where it was found that hypoxia and hypertonicity upregulated NR3a expression. Using short-hairpin (sh) RNA-based knockdown, a stable inner medullary collecting duct (IMCD) cell line was established that had ∼80% decrease in NR3a. Knockdown cells exhibited an increased basal intracellular calcium concentration, reduced cell proliferation, and increased cell death. In addition, NR3a knockdown cells exhibited reduced water transport in response to the addition of vasopressin, suggesting an alteration in aquaporin-2 (AQP2) expression/function. Consistent with this notion, we demonstrate decreased surface expression of glycosylated AQP2 in IMCD cells transfected with NR3a shRNA. To determine whether this also occurred in vivo, we compared AQP2 levels in wild-type vs. in NR3a(-/-) mice. Total AQP2 protein levels in the outer and inner medulla were significantly reduced in knockout mice compared with control mice. Finally, NR3a(-/-) mice showed a significant delay in their ability to increase urine osmolality during water restriction. Thus NR3a may play a renoprotective role in collecting duct cells. Therefore, under conditions that are associated with high vasopressin levels, NR3a, by maintaining low intracellular calcium levels, protects the function of the principal cells to reabsorb water and thereby increase medullary osmolality. | | 21429969
 |
Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex.
Henson, MA; Roberts, AC; Salimi, K; Vadlamudi, S; Hamer, RM; Gilmore, JH; Jarskog, LF; Philpot, BD
Cerebral cortex (New York, N.Y. : 1991)
18
2560-73
2008
Show Abstract
Subunit composition of N-methyl-D-aspartate-type glutamate receptors (NMDARs) dictates their function, yet the ontogenic profiles of human NMDAR subunits from gestation to adulthood have not been determined. We examined NMDAR mRNA and protein development in human dorsolateral prefrontal cortex (DLPFC), an area in which NMDARs are critical for higher cognitive processing and NMDAR hypofunction is hypothesized in schizophrenia. Using quantitative reverse transcriptase-polymerase chain reaction and western blotting, we found NR1 expression begins low prenatally, peaks in adolescence, yet remains high throughout life, suggesting lifelong importance of NMDAR function. In contrast, NR3A levels are low during gestation, surge soon after birth, and decline progressively through adolescence and into adulthood. Because NR3A subunits uniquely attenuate NMDAR-mediated currents, limit calcium influx, and suppress dendritic spine formation, high levels during early childhood may be important for regulating neuroprotection and activity-dependent sculpting of synapses. We also examined whether subunit changes underlie reduced NMDAR activity in schizophrenia. Our results reveal normal NR1 and NR3A protein levels in DLPFC from schizophrenic patients, indicating that NMDAR hypofunction is unlikely to be maintained by gross changes in NR3A-containing NMDARs or overall NMDAR numbers. These data provide insights into NMDAR functions in the developing CNS and will contribute to designing pharmacotherapies for neurological disorders. | | 18296432
 |
NR3 protein expression in trigeminal neurons during postnatal development.
Kohji Ishihama, Jack E Turman
Brain research
1095
12-6
2006
Show Abstract
The N-methyl-d-aspartate (NMDA) receptor plays an important role in the generation of rhythmical oral motor activities. To compliment our previous studies, we examined the developmental regulation of NR3A and NR3B expression in trigeminal motoneurons (Mo5) and mesencephalic trigeminal neurons (Me5). NR3A-immunoreactive neurons were observed at all ages in both nuclei, decreasing in Mo5 and caudal Me5 after P14, and increasing in rostral Me5. NR3B protein expression only emerged in Mo5 after P21-23. Results indicate that NR3A and NR3B expression is differentially regulated between Mo5 and Me5 coincident with the transition from suckling to chewing. | | 16709403
 |
Region specific regulation of NR1 in rhesus monkeys following chronic antipsychotic drug administration.
O'Connor JA, Hasenkamp W, Horman BM, Muly EC, Hemby SE.
Biological psychiatry
60
659-62
2006
Show Abstract
BACKGROUND: Altered NMDA receptor subunit protein levels have been reported in various regions of the schizophrenic brain; however, chronic antipsychotic administration in schizophrenic subjects may confound interpretation. METHODS: The effects of chronic antipsychotic drug administration (haloperidol and clozapine) on protein levels of NR1, NR2A and NR2B proteins were evaluated in the nucleus accumbens (NAc), putamen (PUT), dorsolateral prefrontal cortex (DLPFC), superior temporal gyrus (STG), and entorhinal cortex (EC) of rhesus monkeys using Western blot analysis. RESULTS: Haloperidol administration significantly decreased NR1 expression in the DLPFC. In contrast, NR2B expression was not affected by antipsychotic administration in any brain region examined. NR2A was not reliably detected in any of the brain regions. CONCLUSIONS: Results indicate that the NR1 subunit in the DLPFC may be a substrate for antipsychotic action and that glutamatergic hypofunction in the DLPFC commonly associated with cognitive dysfunction in schizophrenia may be associated with haloperidol administration. | | 16806093
 |
Prenatal development of NMDA receptor composition and function in trigeminal neurons.
Kohji Ishihama, Mikihiko Kogo, Satoshi Wakisaka, Jack E Turman, Kohji Ishihama, Mikihiko Kogo, Satoshi Wakisaka, Jack E Turman
Archives of histology and cytology
68
321-35
2005
Show Abstract
The prenatal development of neural circuits for rhythmical oral-motor behaviors used for feeding is essential for the survival of the newborn mammal. The N-methyl-D-aspartate (NMDA) receptor plays a critical role in brainstem circuits underlying postnatal oral-motor behaviors. To understand a role for the NMDA receptor in the emergence of sucking behavior we conducted physiological and immunohistochemical experiments using fetal rats. Physiology experiments examined the development of the NMDA dose response of the brainstem circuit responsible for generating rhythmical trigeminal activity by recording trigeminal motor outputs using an in vitro preparation. The high dose of NMDA agonist bath application affected the mean cycle duration of rhythmical trigeminal activity (RTA) at both embryonic day (E) 18-19 and E20-21 in comparison with standard concentration of NMDA agonist. NMDA receptor immunohistochemistry studies, using antibodies directed against subunits NR1, NR2A, NR2B, NR3A and NR3B were performed to determine the prenatal regulation of NMDA subunits in trigeminal motoneurons (Mo5), and mesencephalic trigeminal neurons (Me5) between E17 to E20. In Mo5, NR1, NR2A, NR2B and NR3A immunoreactivity was observed throughout the time frame sampled. NR3B immunoreactivity was not observed in Mo5 or Me5. In Mo5, there was a significant decrease in the percentage of NR2B immunoreactive neurons between E17 and E20, and a concurrent increase in the NR2A/NR2B ratio between E17 and E20. In Me5, NR1, NR2A and NR3A immunoreactivity was observed throughout the time frame sampled; a significant decrease in the percentage of NR2A immunoreactive neurons between E17 and E20, and NR3A immunoreactive neurons between E17 and E18 occurred. The timing of subunit changes between E17 and E18 is coincident with the prenatal emergence of rhythmical jaw movements, and in vitro rhythmical trigeminal activity, shown in earlier studies. Our data suggest that NMDA receptor plays an important role in the development and function of prenatal oral-motor circuits. | | 16477151
 |
Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration.
Hemby, SE; Horman, B; Tang, W
Brain research
1064
75-82
2005
Show Abstract
Previous examination of binge cocaine self-administration and 2 week withdrawal from cocaine self-administration on ionotropic glutamate receptor subunit (iGluRs) protein levels revealed significant alterations in iGluR protein levels that differed between the mesocorticolimbic and nigrostriatal pathways. The present study was undertaken to extend the examination of cocaine-induced alterations in iGluR protein expression by assessing the effects of acute withdrawal (15-16 h) from limited access cocaine self-administration (8 h/day, 15 days). Western blotting was used to compare levels of iGluR protein expression (NR1-3B, GluR1-7, KA2) in the mesolimbic (ventral tegmental area, VTA; nucleus accumbens, NAc; and prefrontal cortex, PFC) and nigrostriatal pathways (substantia nigra, SN and dorsal caudate-putamen, CPu). Within the mesolimbic pathway, reductions were observed in NR1 and GluR5 immunoreactivity in the VTA although no significant alterations were observed in any iGluR subunits in the NAc. In the PFC, NR1 was significantly upregulated while GluR2/3, GluR4, GluR5, GluR6/7, and KA2 were decreased. Within the nigrostriatal pathway, NR1, NR2A, NR2B, GluR1, GluR6/7 and KA2 were increased in the dorsal CPu, whereas no significant changes were observed in the SN. The results demonstrate region- and pathway-specific alterations in iGluR subunit expression following limited cocaine self-administration and suggest the importance for the activation of pathways that are substrates of the reinforcing and motoric effects of cocaine. | | 16277980
 |
Structure and function of glutamate receptor ion channels.
Mayer, Mark L and Armstrong, Neali
Annu. Rev. Physiol., 66: 161-81 (2004)
2004
Show Abstract
A vast number of proteins are involved in synaptic function. Many have been cloned and their functional role defined with varying degrees of success, but their number and complexity currently defy any molecular understanding of the physiology of synapses. A beacon of success in this medieval era of synaptic biology is an emerging understanding of the mechanisms underlying the activity of the neurotransmitter receptors for glutamate. Largely as a result of structural studies performed in the past three years we now have a mechanistic explanation for the activation of channel gating by agonists and partial agonists; the process of desensitization, and its block by allosteric modulators, is also mostly explained; and the basis of receptor subtype selectivity is emerging with clarity as more and more structures are solved. In the space of months we have gone from cartoons of postulated mechanisms to hard fact. It is anticipated that this level of understanding will emerge for other synaptic proteins in the coming decade. | | 14977400
 |