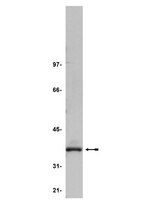
Antibodies recognizing the C terminus of PP2A catalytic subunit are unsuitable for evaluating PP2A activity and holoenzyme composition
Ingrid E Frohner, Ingrid Mudrak, Stephanie Kronlachner, Stefan Schüchner, Egon Ogris<br />Affiliations<br />1. Center for Medical Biochemistry, Max Perutz Labs, Vienna BioCenter, Medical University of Vienna, Dr. Bohr-Gasse 9, A-1030 Vienna, Austria.<br />2. Center for Medical Biochemistry, Max Perutz Labs, Vienna BioCenter, Medical University of Vienna, Dr. Bohr-Gasse 9, A-1030 Vienna, Austria. egon.ogris@meduniwien.ac.at.
Science Signaling
2020
Show Abstract
The methyl-esterification of the C-terminal leucine of the protein phosphatase 2A (PP2A) catalytic (C) subunit is essential for the assembly of specific trimeric PP2A holoenzymes, and this region of the C subunit also contains two threonine and tyrosine phosphorylation sites. Most commercial antibodies—including the monoclonal antibody 1D6 that is part of a frequently used, commercial phosphatase assay kit—are directed toward the C terminus of the C subunit, raising questions as to their ability to recognize methylated and phosphorylated forms of the enzyme. Here, we tested several PP2A C antibodies, including monoclonal antibodies 1D6, 7A6, G-4, and 52F8 and the polyclonal antibody 2038 for their ability to specifically detect PP2A in its various modified forms, as well as to coprecipitate regulatory subunits. The tested antibodies preferentially recognized the nonmethylated form of the enzyme, and they did not coimmunoprecipitate trimeric holoenzymes containing the regulatory subunits B or B′, an issue that precludes their use to monitor PP2A holoenzyme activity. Furthermore, some of the antibodies also recognized the phosphatase PP4, demonstrating a lack of specificity for PP2A. Together, these findings suggest that reinterpretation of the data generated by using these reagents is required. | | 31992581
 |
The B55α regulatory subunit of protein phosphatase 2A mediates fibroblast growth factor-induced p107 dephosphorylation and growth arrest in chondrocytes.
Kolupaeva, V; Daempfling, L; Basilico, C
Molecular and cellular biology
33
2865-78
2013
Show Abstract
Fibroblast growth factor (FGF)-induced growth arrest of chondrocytes is a unique cell type-specific response which contrasts with the proliferative response of most cell types and underlies several genetic skeletal disorders caused by activating FGF receptor (FGFR) mutations. We have shown that one of the earliest key events in FGF-induced growth arrest is dephosphorylation of the retinoblastoma protein (Rb) family member p107 by protein phosphatase 2A (PP2A), a ubiquitously expressed multisubunit phosphatase. In this report, we show that the PP2A-B55α holoenzyme (PP2A containing the B55α subunit) is responsible for this phenomenon. Only the B55α (55-kDa regulatory subunit, alpha isoform) regulatory subunit of PP2A was able to bind p107, and this interaction was induced by FGF in chondrocytes but not in other cell types. Small interfering RNA (siRNA)-mediated knockdown of B55α prevented p107 dephosphorylation and FGF-induced growth arrest of RCS (rat chondrosarcoma) chondrocytes. Importantly, the B55α subunit bound with higher affinity to dephosphorylated p107. Since the p107 region interacting with B55α is also the site of cyclin-dependent kinase (CDK) binding, B55α association may also prevent p107 phosphorylation by CDKs. FGF treatment induces dephosphorylation of the B55α subunit itself on several serine residues that drastically increases the affinity of B55α for the PP2A A/C dimer and p107. Together these observations suggest a novel mechanism of p107 dephosphorylation mediated by activation of PP2A through B55α dephosphorylation. This mechanism might be a general signal transduction pathway used by PP2A to initiate cell cycle arrest when required by external signals. | | 23716589
 |
Modeling initiation of Ewing sarcoma in human neural crest cells.
von Levetzow, C; Jiang, X; Gwye, Y; von Levetzow, G; Hung, L; Cooper, A; Hsu, JH; Lawlor, ER
PloS one
6
e19305
2011
Show Abstract
Ewing sarcoma family tumors (ESFT) are aggressive bone and soft tissue tumors that express EWS-ETS fusion genes as driver mutations. Although the histogenesis of ESFT is controversial, mesenchymal (MSC) and/or neural crest (NCSC) stem cells have been implicated as cells of origin. For the current study we evaluated the consequences of EWS-FLI1 expression in human embryonic stem cell-derived NCSC (hNCSC). Ectopic expression of EWS-FLI1 in undifferentiated hNCSC and their neuro-mesenchymal stem cell (hNC-MSC) progeny was readily tolerated and led to altered expression of both well established as well as novel EWS-FLI1 target genes. Importantly, whole genome expression profiling studies revealed that the molecular signature of established ESFT is more similar to hNCSC than any other normal tissue, including MSC, indicating that maintenance or reactivation of the NCSC program is a feature of ESFT pathogenesis. Consistent with this hypothesis, EWS-FLI1 induced hNCSC genes as well as the polycomb proteins BMI-1 and EZH2 in hNC-MSC. In addition, up-regulation of BMI-1 was associated with avoidance of cellular senescence and reversible silencing of p16. Together these studies confirm that, unlike terminally differentiated cells but consistent with bone marrow-derived MSC, NCSC tolerate expression of EWS-FLI1 and ectopic expression of the oncogene initiates transition to an ESFT-like state. In addition, to our knowledge this is the first demonstration that EWS-FLI1-mediated induction of BMI-1 and epigenetic silencing of p16 might be critical early initiating events in ESFT tumorigenesis. Full Text Article | | 21559395
 |
The role of organic anion transporting polypeptides (OATPs/SLCOs) in the toxicity of different microcystin congeners in vitro: a comparison of primary human hepatocytes and OATP-transfected HEK293 cells.
Fischer A, Hoeger SJ, Stemmer K, Feurstein DJ, Knobeloch D, Nussler A, Dietrich DR
Toxicol Appl Pharmacol
245
9-20. Epub 2010 Feb 17.
2010
Show Abstract
Cellular uptake of microcystins (MCs), a family of cyclic cyanobacterial heptapeptide toxins, occurs via specific organic anion transporting polypeptides (OATPs), where MCs inhibit serine/threonine-specific protein phosphatase (PP). Despite comparable PP-inhibitory capacity, MCs differ greatly in their acute toxicity, thus raising the question whether this discrepancy results from MC-specific toxikokinetic rather than toxicodynamic differences. OATP-mediated uptake of MC congeners MCLR, -RR, -LW and -LF was compared in primary human hepatocytes and HEK293 cells stably expressing recombinant human OATP1B1/SLCO1B1 and OATP1B3/SLCO1B3 in the presence/absence of OATP substrates taurocholate (TC) and bromosulfophthalein (BSP) and measuring PP-inhibition and cytotoxicity. Control vector expressing HEK293 were resistant to MC cytotoxicity, while TC and BSP competition experiments reduced MC cytotoxicity in HEK293-OATP transfectants, thus confirming the requirement of OATPs for trans-membrane transport. Despite comparable PP-inhibiting capabilities, MCLW and -LF elicited cytotoxic effects at lower equimolar concentrations than MCLR and MCRR, hence suggesting congener selective transport into HEK293-OATP transfectants and primary human hepatocytes. Primary human hepatocytes appeared one order of magnitude more sensitive to MC congeners than the corresponding HEK293 -OATP transfectants. Although the latter maybe due to a much lower level of PPs in primary human hepatocytes, the presence of OATPs other than 1B1 or 1B3 may have added to an increased uptake of MCs. In view of the high sensitivity of human hepatocytes and currently MCLR-only based risk calculations, the actual risk of human MC-intoxication and ensuing liver damage could be underestimated in freshwater cyanobacterial blooms where MCLW and-LF predominate. | | 20171238
 |
Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells.
Ke, Y; Lei, M; Collins, TP; Rakovic, S; Mattick, PA; Yamasaki, M; Brodie, MS; Terrar, DA; Solaro, RJ
Circulation research
100
1317-27
2007
Show Abstract
Phosphorylation of ion channels plays an important role in the regulation of cardiac function, but signaling mechanisms controlling dephosphorylation are not well understood. We have tested the hypothesis that p(21)-activated kinase-1 (Pak1), a serine-threonine protein kinase regulated by Ras-related small G proteins, regulates sinoatrial node (SAN) ion channel activity through a mechanism involving protein phosphatase 2A. We report a novel role of Pak1-mediated signaling in attenuating isoproterenol-induced enhancement of L-type Ca(2+) current (I(CaL)) and delayed rectifier potassium current (I(K)) in guinea pig SAN pacemaker cells. We demonstrate that in guinea pig SAN: (1) there is abundant expression of endogenous Pak1 in pacemaker cells; (2) expression of constitutively active Pak1 depresses isoproterenol-induced upregulation of I(CaL) and I(K); (3) inhibition of protein phosphatase 2A increases the enhancement of I(K) and I(CaL) by isoproterenol in Ad-Pak1-infected cells; (4) protein phosphatase 2A coimmunoprecipitates with endogenous Pak1 in SAN tissue; and (5) expression of constitutively active Pak1 suppresses the chronotropic action of isoproterenol on pacemaker activity of intact SAN preparations. In conclusion, our data demonstrate that a Pak1 signaling pathway exists in cardiac pacemaker cells and that this novel pathway plays a role in the regulation of ion channel activity. | Immunofluorescence | 17413045
 |
Loss of protein phosphatase 2A expression correlates with phosphorylation of DP-1 and reversal of dysplasia through differentiation in a conditional mouse model of cancer progression.
Maddalena T Tilli, Shawnté L Hudgins, M Silvina Frech, Ewa D Halama, Jean-Pierre Renou, Priscilla A Furth
Cancer research
63
7668-73
2003
Show Abstract
A conditional mouse model of time-dependent dysplasia reversal demonstrated that reversal and differentiation of dysplastic salivary gland tissue at the 4-month reversible stage was characterized by the appearance of a phosphorylated slower mobility form of Differentiation Related Transcription Factor 1-polypeptide-1 that was correlated with cellular differentiation. The phosphorylated form of DP-1 was not found at the 7-month irreversible stage or in adenocarcinomas. At the 4-month reversible stage, protein phosphatase 2A expression was down-regulated coincident with loss of oncogene expression, whereas PP2A expression persisted at the 7-month irreversible stage. Results are consistent with the hypothesis that persistent PP2A expression prevented the appearance of the phosphorylated form of DP-1 required for cellular differentiation and reversal of dysplasia after loss of oncogene expression. | | 14633688
 |
Identification of a subunit of a novel Kleisin-beta/SMC complex as a potential substrate of protein phosphatase 2A
Yeong, F. M., et al
Curr Biol, 13:2058-64 (2003)
2003
| Immunoprecipitation | 14653995
 |
Altering the holoenzyme composition and substrate specificity of protein phosphatase 2A
Fellner, T., et al
Methods Enzymol, 366:187-203 (2003)
2003
| Immunoprecipitation | 14674250
 |
ATM-dependent dissociation of B55 regulatory subunit from nuclear PP2A in response to ionizing radiation
Guo, C. Y., et al
J Biol Chem, 277:4839-44 (2002)
2002
| Immunoblotting (Western) | 11723136
 |
A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A.
Ogris, E, et al.
J. Biol. Chem., 274: 14382-91 (1999)
1999
Show Abstract
Carboxymethylation of proteins is a highly conserved means of regulation in eukaryotic cells. The protein phosphatase 2A (PP2A) catalytic (C) subunit is reversibly methylated at its carboxyl terminus by specific methyltransferase and methylesterase enzymes which have been purified, but not cloned. Carboxymethylation affects PP2A activity and varies during the cell cycle. Here, we report that substitution of glutamine for either of two putative active site histidines in the PP2A C subunit results in inactivation of PP2A and formation of stable complexes between PP2A and several cellular proteins. One of these cellular proteins, herein named protein phosphatase methylesterase-1 (PME-1), was purified and microsequenced, and its cDNA was cloned. PME-1 is conserved from yeast to human and contains a motif found in lipases having a catalytic triad-activated serine as their active site nucleophile. Bacterially expressed PME-1 demethylated PP2A C subunit in vitro, and okadaic acid, a known inhibitor of the PP2A methylesterase, inhibited this reaction. To our knowledge, PME-1 represents the first mammalian protein methylesterase to be cloned. Several lines of evidence indicate that, although there appears to be a role for C subunit carboxyl-terminal amino acids in PME-1 binding, amino acids other than those at the extreme carboxyl terminus of the C subunit also play an important role in PME-1 binding to a catalytically inactive mutant. | | 10318862
 |