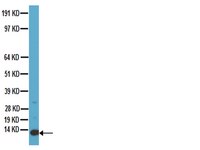
Deletion of a kinesin I motor unmasks a mechanism of homeostatic branching control by neurotrophin-3.
Auer, TO; Xiao, T; Bercier, V; Gebhardt, C; Duroure, K; Concordet, JP; Wyart, C; Suster, M; Kawakami, K; Wittbrodt, J; Baier, H; Del Bene, F
eLife
4
2015
Show Abstract
Development and function of highly polarized cells such as neurons depend on microtubule-associated intracellular transport, but little is known about contributions of specific molecular motors to the establishment of synaptic connections. In this study, we investigated the function of the Kinesin I heavy chain Kif5aa during retinotectal circuit formation in zebrafish. Targeted disruption of Kif5aa does not affect retinal ganglion cell differentiation, and retinal axons reach their topographically correct targets in the tectum, albeit with a delay. In vivo dynamic imaging showed that anterograde transport of mitochondria is impaired, as is synaptic transmission. Strikingly, disruption of presynaptic activity elicits upregulation of Neurotrophin-3 (Ntf3) in postsynaptic tectal cells. This in turn promotes exuberant branching of retinal axons by signaling through the TrkC receptor (Ntrk3). Thus, our study has uncovered an activity-dependent, retrograde signaling pathway that homeostatically controls axonal branching. | | | 26076409
 |
Peroxisome proliferator-activated receptor alpha plays a crucial role in behavioral repetition and cognitive flexibility in mice.
D'Agostino, G; Cristiano, C; Lyons, DJ; Citraro, R; Russo, E; Avagliano, C; Russo, R; Raso, GM; Meli, R; De Sarro, G; Heisler, LK; Calignano, A
Molecular metabolism
4
528-36
2015
Show Abstract
Nuclear peroxisome proliferator activated receptor-α (PPAR-α) plays a fundamental role in the regulation of lipid homeostasis and is the target of medications used to treat dyslipidemia. However, little is known about the role of PPAR-α in mouse behavior.To investigate the function of Ppar-α in cognitive functions, a behavioral phenotype analysis of mice with a targeted genetic disruption of Ppar-α was performed in combination with neuroanatomical, biochemical and pharmacological manipulations. The therapeutic exploitability of PPAR-α was probed in mice using a pharmacological model of psychosis and a genetic model (BTBR T + tf/J) exhibiting a high rate of repetitive behavior.An unexpected role for brain Ppar-α in the regulation of cognitive behavior in mice was revealed. Specifically, we observed that Ppar-α genetic perturbation promotes rewiring of cortical and hippocampal regions and a behavioral phenotype of cognitive inflexibility, perseveration and blunted responses to psychomimetic drugs. Furthermore, we demonstrate that the antipsychotic and autism spectrum disorder (ASD) medication risperidone ameliorates the behavioral profile of Ppar-α deficient mice. Importantly, we reveal that pharmacological PPAR-α agonist treatment in mice improves behavior in a pharmacological model of ketamine-induced behavioral dysinhibition and repetitive behavior in BTBR T + tf/J mice.Our data indicate that Ppar-α is required for normal cognitive function and that pharmacological stimulation of PPAR-α improves cognitive function in pharmacological and genetic models of impaired cognitive function in mice. These results thereby reveal an unforeseen therapeutic application for a class of drugs currently in human use. | | | 26137440
 |
Processing of visually evoked innate fear by a non-canonical thalamic pathway.
Wei, P; Liu, N; Zhang, Z; Liu, X; Tang, Y; He, X; Wu, B; Zhou, Z; Liu, Y; Li, J; Zhang, Y; Zhou, X; Xu, L; Chen, L; Bi, G; Hu, X; Xu, F; Wang, L
Nature communications
6
6756
2015
Show Abstract
The ability of animals to respond to life-threatening stimuli is essential for survival. Although vision provides one of the major sensory inputs for detecting threats across animal species, the circuitry underlying defensive responses to visual stimuli remains poorly defined. Here, we investigate the circuitry underlying innate defensive behaviours elicited by predator-like visual stimuli in mice. Our results demonstrate that neurons in the superior colliculus (SC) are essential for a variety of acute and persistent defensive responses to overhead looming stimuli. Optogenetic mapping revealed that SC projections to the lateral posterior nucleus (LP) of the thalamus, a non-canonical polymodal sensory relay, are sufficient to mimic visually evoked fear responses. In vivo electrophysiology experiments identified a di-synaptic circuit from SC through LP to the lateral amygdale (Amg), and lesions of the Amg blocked the full range of visually evoked defensive responses. Our results reveal a novel collicular-thalamic-Amg circuit important for innate defensive responses to visual threats. | | | 25854147
 |
Fgf signaling controls the telencephalic distribution of Fgf-expressing progenitors generated in the rostral patterning center.
Hoch, RV; Clarke, JA; Rubenstein, JL
Neural development
10
8
2015
Show Abstract
The rostral patterning center (RPC) secretes multiple fibroblast growth factors (Fgfs) essential for telencephalon growth and patterning. Fgf expression patterns suggest that they mark functionally distinct RPC subdomains. We generated Fgf8(CreER) and Fgf17(CreER) mice and used them to analyze the lineages of Fgf8- versus Fgf17-expressing RPC cells.Both lineages contributed to medial structures of the rostroventral telencephalon structures including the septum and medial prefrontral cortex. In addition, RPC-derived progenitors were observed in other regions of the early telencephalic neuroepithelium and generated neurons in the olfactory bulb, neocortex, and basal ganglia. Surprisingly, Fgf8(+) RPC progenitors generated the majority of basal ganglia cholinergic neurons. Compared to the Fgf8 lineage, the Fgf17 lineage was more restricted in its early dispersion and its contributions to the telencephalon. Mutant studies suggested that Fgf8 and Fgf17 restrict spread of RPC progenitor subpopulations.We identified the RPC as an important source of progenitors that contribute broadly to the telencephalon and found that two molecularly distinct progenitor subtypes in the RPC make different contributions to the developing forebrain. | | | 25889070
 |
Differential expression of hyperpolarization-activated cyclic nucleotide-gated channel subunits during hippocampal development in the mouse.
Seo, H; Seol, MJ; Lee, K
Molecular brain
8
13
2015
Show Abstract
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels help control the rhythmic activation of pacemaker neurons during brain development. However, little is known about the timing and cell type specificity of the expression of HCN isoforms during development of the hippocampus.Here we examined the developmental expression of the brain-enriched HCN1, HCN2, and HCN4 isoforms of HCN channels in mouse hippocampus from embryonic to postnatal stages. All these isoforms were expressed abundantly in the hippocampus at embryonic day 14.5 and postnatal day 0. Each HCN channel isoform showed subfield-specific expression within the hippocampus from postnatal day 7, and only HCN4 was found in glial cells in the stratum lacunosum moleculare at this developmental stage. At postnatal days 21 and 56, all HCN isoforms were strongly expressed in the stratum lacunosum moleculare and the stratum pyramidale of the Cornu Ammonis (CA), as well as in the hilus of the dentate gyrus, but not in the subgranular zone. Furthermore, the immunolabeling for all these isoforms was colocalized with parvalbumin immunolabeling in interneurons of the CA field and in the dentate gyrus.Our mapping data showing the temporal and spatial changes in the expression of HCN channels suggest that HCN1, HCN2, and HCN4 subunits may have distinct physiological roles in the developing hippocampus. | | | 25761792
 |
Visual recognition memory, manifested as long-term habituation, requires synaptic plasticity in V1.
Cooke, SF; Komorowski, RW; Kaplan, ES; Gavornik, JP; Bear, MF
Nature neuroscience
18
262-71
2015
Show Abstract
Familiarity with stimuli that bring neither reward nor punishment, manifested through behavioral habituation, enables organisms to detect novelty and devote cognition to important elements of the environment. Here we describe in mice a form of long-term behavioral habituation to visual grating stimuli that is selective for stimulus orientation. Orientation-selective habituation (OSH) can be observed both in exploratory behavior in an open arena and in a stereotyped motor response to visual stimuli in head-restrained mice. We found that the latter behavioral response, termed a 'vidget', requires V1. Parallel electrophysiological recordings in V1 revealed that plasticity, in the form of stimulus-selective response potentiation (SRP), occurred in layer 4 of V1 as OSH developed. Local manipulations of V1 that prevented and reversed electrophysiological modifications likewise prevented and reversed memory demonstrated behaviorally. These findings suggest that a form of long-term visual recognition memory is stored via synaptic plasticity in primary sensory cortex. | | | 25599221
 |
OTX2 Transcription Factor Controls Regional Patterning within the Medial Ganglionic Eminence and Regional Identity of the Septum.
Hoch, RV; Lindtner, S; Price, JD; Rubenstein, JL
Cell reports
12
482-94
2015
Show Abstract
The Otx2 homeodomain transcription factor is essential for gastrulation and early neural development. We generated Otx2 conditional knockout (cKO) mice to investigate its roles in telencephalon development after neurulation (approximately embryonic day 9.0). We conducted transcriptional profiling and in situ hybridization to identify genes de-regulated in Otx2 cKO ventral forebrain. In parallel, we used chromatin immunoprecipitation sequencing to identify enhancer elements, the OTX2 binding motif, and de-regulated genes that are likely direct targets of OTX2 transcriptional regulation. We found that Otx2 was essential in septum specification, regulation of Fgf signaling in the rostral telencephalon, and medial ganglionic eminence (MGE) patterning, neurogenesis, and oligodendrogenesis. Within the MGE, Otx2 was required for ventral, but not dorsal, identity, thus controlling the production of specific MGE derivatives. | | | 26166575
 |
Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding.
De Andrade, PB; Neff, LA; Strosova, MK; Arsenijevic, D; Patthey-Vuadens, O; Scapozza, L; Montani, JP; Ruegg, UT; Dulloo, AG; Dorchies, OM
Frontiers in physiology
6
254
2015
Show Abstract
Weight regain after caloric restriction results in accelerated fat storage in adipose tissue. This catch-up fat phenomenon is postulated to result partly from suppressed skeletal muscle thermogenesis, but the underlying mechanisms are elusive. We investigated whether the reduced rate of skeletal muscle contraction-relaxation cycle that occurs after caloric restriction persists during weight recovery and could contribute to catch-up fat. Using a rat model of semistarvation-refeeding, in which fat recovery is driven by suppressed thermogenesis, we show that contraction and relaxation of leg muscles are slower after both semistarvation and refeeding. These effects are associated with (i) higher expression of muscle deiodinase type 3 (DIO3), which inactivates tri-iodothyronine (T3), and lower expression of T3-activating enzyme, deiodinase type 2 (DIO2), (ii) slower net formation of T3 from its T4 precursor in muscles, and (iii) accumulation of slow fibers at the expense of fast fibers. These semistarvation-induced changes persisted during recovery and correlated with impaired expression of transcription factors involved in slow-twitch muscle development. We conclude that diminished muscle thermogenesis following caloric restriction results from reduced muscle T3 levels, alteration in muscle-specific transcription factors, and fast-to-slow fiber shift causing slower contractility. These energy-sparing effects persist during weight recovery and contribute to catch-up fat. | | | 26441673
 |
Human breast cancer metastases to the brain display GABAergic properties in the neural niche.
Neman, J; Termini, J; Wilczynski, S; Vaidehi, N; Choy, C; Kowolik, CM; Li, H; Hambrecht, AC; Roberts, E; Jandial, R
Proceedings of the National Academy of Sciences of the United States of America
111
984-9
2014
Show Abstract
Dispersion of tumors throughout the body is a neoplastic process responsible for the vast majority of deaths from cancer. Despite disseminating to distant organs as malignant scouts, most tumor cells fail to remain viable after their arrival. The physiologic microenvironment of the brain must become a tumor-favorable microenvironment for successful metastatic colonization by circulating breast cancer cells. Bidirectional interplay of breast cancer cells and native brain cells in metastasis is poorly understood and rarely studied. We had the rare opportunity to investigate uncommonly available specimens of matched fresh breast-to-brain metastases tissue and derived cells from patients undergoing neurosurgical resection. We hypothesized that, to metastasize, breast cancers may escape their normative genetic constraints by accommodating and coinhabiting the neural niche. This acquisition or expression of brain-like properties by breast cancer cells could be a malignant adaptation required for brain colonization. Indeed, we found breast-to-brain metastatic tissue and cells displayed a GABAergic phenotype similar to that of neuronal cells. The GABAA receptor, GABA transporter, GABA transaminase, parvalbumin, and reelin were all highly expressed in breast cancer metastases to the brain. Proliferative advantage was conferred by the ability of breast-to-brain metastases to take up and catabolize GABA into succinate with the resultant formation of NADH as a biosynthetic source through the GABA shunt. The results suggest that breast cancers exhibit neural characteristics when occupying the brain microenvironment and co-opt GABA as an oncometabolite. | | Human | 24395782
 |
Two cases of multinodular and vacuolating neuronal tumour.
Bodi, I; Curran, O; Selway, R; Elwes, R; Burrone, J; Laxton, R; Al-Sarraj, S; Honavar, M
Acta neuropathologica communications
2
7
2014
Show Abstract
An unusual multinodular and vacuolating neuronal tumour (MVNT) has been described in the cerebral hemispheres of ten patients with adult-onset seizures. We report the findings in two cases with similar features, a surgical resection and the other an autopsy specimen.Case 1, a 34-year-old female, underwent surgical resection for a multinodular non-enhancing frontal white matter lesion causing intractable epilepsy. Case 2, presented with motor neurone disease (MND) at the age of 71 and MRI scanning revealed extensive multinodular non-enhancing white matter lesions in the temporal lobe. There was no history of epilepsy and post mortem histology confirmed MND.Macroscopically multiple small grey well-formed, discrete and coalescent nodules were seen in the deep cortex and subcortical white matter. On histology, mature-looking neurons with large cytoplasmic vacuoles were distributed in a fibrillary background, where vacuoles were also noted. In the resected tumour scattered oligodendroglia-like cells were present. No ganglion cells were seen. The vacuolated cells exhibited immunopositivity for synaptophysin, HuC/HuD and p62 but were negative for NeuN, neurofilament, GFAP, IDH1, nestin and CD34. Electron microscopy showed non-membrane bound cytoplasmic vacuoles in the neurons and in some neuronal processes. The seizures recurred in Case 1.Some clinicopathological features of this lesion suggest a possible relationship with dysembryoplastic neuroepithelial tumour (DNT) although the morphological features are not typical of DNT. Case 2 demonstrates that MVNT may remain asymptomatic. | | | 24444358
 |