Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in Drosophila.
Zhou, Q; Bachtrog, D
PLoS genetics
11
e1005331
2015
Show Abstract
Sex chromosomes evolve distinctive types of chromatin from a pair of ancestral autosomes that are usually euchromatic. In Drosophila, the dosage-compensated X becomes enriched for hyperactive chromatin in males (mediated by H4K16ac), while the Y chromosome acquires silencing heterochromatin (enriched for H3K9me2/3). Drosophila autosomes are typically mostly euchromatic but the small dot chromosome has evolved a heterochromatin-like milieu (enriched for H3K9me2/3) that permits the normal expression of dot-linked genes, but which is different from typical pericentric heterochromatin. In Drosophila busckii, the dot chromosomes have fused to the ancestral sex chromosomes, creating a pair of 'neo-sex' chromosomes. Here we collect genomic, transcriptomic and epigenomic data from D. busckii, to investigate the evolutionary trajectory of sex chromosomes from a largely heterochromatic ancestor. We show that the neo-sex chromosomes formed less than 1 million years ago, but nearly 60% of neo-Y linked genes have already become non-functional. Expression levels are generally lower for the neo-Y alleles relative to their neo-X homologs, and the silencing heterochromatin mark H3K9me2, but not H3K9me3, is significantly enriched on silenced neo-Y genes. Despite rampant neo-Y degeneration, we find that the neo-X is deficient for the canonical histone modification mark of dosage compensation (H4K16ac), relative to autosomes or the compensated ancestral X chromosome, possibly reflecting constraints imposed on evolving hyperactive chromatin in an originally heterochromatic environment. Yet, neo-X genes are transcriptionally more active in males, relative to females, suggesting the evolution of incipient dosage compensation on the neo-X. Our data show that Y degeneration proceeds quickly after sex chromosomes become established through genomic and epigenetic changes, and are consistent with the idea that the evolution of sex-linked chromatin is influenced by its ancestral configuration. | | | 26114585
 |
Mutation of histone H3 serine 86 disrupts GATA factor Ams2 expression and precise chromosome segregation in fission yeast.
Lim, KK; Ong, TY; Tan, YR; Yang, EG; Ren, B; Seah, KS; Yang, Z; Tan, TS; Dymock, BW; Chen, ES
Scientific reports
5
14064
2015
Show Abstract
Eukaryotic genomes are packed into discrete units, referred to as nucleosomes, by organizing around scaffolding histone proteins. The interplay between these histones and the DNA can dynamically regulate the function of the chromosomal domain. Here, we interrogated the function of a pair of juxtaposing serine residues (S86 and S87) that reside within the histone fold of histone H3. We show that fission yeast cells expressing a mutant histone H3 disrupted at S86 and S87 (hht2-S86AS87A) exhibited unequal chromosome segregation, disrupted transcriptional silencing of centromeric chromatin, and reduced expression of Ams2, a GATA-factor that regulates localization of the centromere-specific histone H3 variant CENP-A. We found that overexpression of ams2(+) could suppress the chromosome missegregation phenotype that arose in the hht2-S86AS87A mutant. We further demonstrate that centromeric localization of SpCENP-A(cnp1-1) was significantly compromised in hht2-S86AS87A, suggesting synergism between histone H3 and the centromere-targeting domain of SpCENP-A. Taken together, our work presents evidence for an uncharacterized serine residue in fission yeast histone H3 that affects centromeric integrity via regulating the expression of the SpCENP-A-localizing Ams2 protein. [173/200 words]. | | | 26369364
 |
TP53 engagement with the genome occurs in distinct local chromatin environments via pioneer factor activity.
Sammons, MA; Zhu, J; Drake, AM; Berger, SL
Genome research
25
179-88
2015
Show Abstract
Despite overwhelming evidence that transcriptional activation by TP53 is critical for its tumor suppressive activity, the mechanisms by which TP53 engages the genome in the context of chromatin to activate transcription are not well understood. Using a compendium of novel and existing genome-wide data sets, we examined the relationship between TP53 binding and the dynamics of the local chromatin environment. Our analysis revealed three distinct categories of TP53 binding events that differ based on the dynamics of the local chromatin environment. The first class of TP53 binding events occurs near transcriptional start sites (TSS) and is defined by previously characterized promoter-associated chromatin modifications. The second class comprises a large cohort of preestablished, promoter-distal enhancer elements that demonstrates dynamic histone acetylation and transcription upon TP53 binding. The third class of TP53 binding sites is devoid of classic chromatin modifications and, remarkably, fall within regions of inaccessible chromatin, suggesting that TP53 has intrinsic pioneer factor activity and binds within structurally inaccessible regions of chromatin. Intriguingly, these inaccessible TP53 binding sites feature several enhancer-like properties in cell types within the epithelial lineage, indicating that TP53 binding events include a group of "proto-enhancers" that become active enhancers given the appropriate cellular context. These data indicate that TP53, along with TP63, may act as pioneer factors to specify epithelial enhancers. Further, these findings suggest that rather than following a global cell-type invariant stress response program, TP53 may tune its response based on the lineage-specific epigenomic landscape. | | | 25391375
 |
LSD1 regulates pluripotency of embryonic stem/carcinoma cells through histone deacetylase 1-mediated deacetylation of histone H4 at lysine 16.
Yin, F; Lan, R; Zhang, X; Zhu, L; Chen, F; Xu, Z; Liu, Y; Ye, T; Sun, H; Lu, F; Zhang, H
Molecular and cellular biology
34
158-79
2014
Show Abstract
LSD1 is essential for the maintenance of pluripotency of embryonic stem (ES) or embryonic carcinoma/teratocarcinoma (EC) cells. We have previously developed novel LSD1 inhibitors that selectively inhibit ES/EC cells. However, the critical targets of LSD1 remain unclear. Here, we found that LSD1 interacts with histone deacetylase 1 (HDAC1) to regulate the proliferation of ES/EC cells through acetylation of histone H4 at lysine 16 (H4K16), which we show is a critical substrate of HDAC1. The LSD1 demethylase and HDAC1 deacetylase activities were both inactivated if one of them in the complex was chemically inhibited in ES/EC cells or in reconstituted protein complexes. Loss of HDAC1 phenocopied the selective growth-inhibitory effects and increased the levels of H3K4 methylation and H4K16 acetylation of LSD1 inactivation on ES/EC cells. Reduction of acetylated H4K16 by ablation of the acetyltransferase males absent on the first (MOF) is sufficient to rescue the growth inhibition induced by LSD1 inactivation. While LSD1 or HDAC1 inactivation caused the downregulation of Sox2 and Oct4 and induction of differentiation genes, such as FOXA2 or BMP2, depletion of MOF restored the levels of Sox2, Oct4, and FoxA2 in LSD1-deficient cells. Our studies reveal a novel mechanism by which LSD1 acts through the HDAC1- and MOF-mediated regulation of H4K16 acetylation to maintain the pluripotency of ES/EC cells. | | | 24190971
 |
The onset of C. elegans dosage compensation is linked to the loss of developmental plasticity.
Custer, LM; Snyder, MJ; Flegel, K; Csankovszki, G
Developmental biology
385
279-90
2014
Show Abstract
Dosage compensation (DC) equalizes X-linked gene expression between sexes. In Caenorhabditis elegans, the dosage compensation complex (DCC) localizes to both X chromosomes in hermaphrodites and downregulates gene expression 2-fold. The DCC first localizes to hermaphrodite X chromosomes at the 30-cell stage, coincident with a developmental transition from plasticity to differentiation. To test whether DC onset is linked to loss of developmental plasticity, we established a timeline for the accumulation of DC-mediated chromatin features on X (depletion of histone H4 lysine 16 acetylation (H4K16ac) and enrichment of H4K20 monomethylation (H4K20me1)) in both wild type and developmentally delayed embryos. Surprisingly, we found that H4K16ac is depleted from the X even before the 30-cell stage in a DCC-independent manner. This depletion requires the activities of MES-2, MES-3, and MES-6 (a complex similar to the Polycomb Repressive Complex 2), and MES-4. By contrast, H4K20me1 becomes enriched on X chromosomes several cell cycles after DCC localization to the X, suggesting that it is a late mark in DC. MES-2 also promotes differentiation, and mes-2 mutant embryos exhibit prolonged developmental plasticity. Consistent with the hypothesis that the onset of DC is linked to differentiation, DCC localization and H4K20me1 accumulation on the X chromosomes are delayed in mes mutant hermaphrodite embryos. Furthermore, the onset of hermaphrodite-specific transcription of sdc-2 (which triggers DC) is delayed in mes-2 mutants. We propose that as embryonic blastomeres lose their developmental plasticity, hermaphrodite X chromosomes transition from a MES protein-regulated state to DCC-mediated repression. | Immunofluorescence | | 24252776
 |
Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory.
Hait, NC; Wise, LE; Allegood, JC; O'Brien, M; Avni, D; Reeves, TM; Knapp, PE; Lu, J; Luo, C; Miles, MF; Milstien, S; Lichtman, AH; Spiegel, S
Nature neuroscience
17
971-80
2014
Show Abstract
FTY720 (fingolimod), an FDA-approved drug for treatment of multiple sclerosis, has beneficial effects in the CNS that are not yet well understood, independent of its effects on immune cell trafficking. We show that FTY720 enters the nucleus, where it is phosphorylated by sphingosine kinase 2 (SphK2), and that nuclear FTY720-P binds and inhibits class I histone deacetylases (HDACs), enhancing specific histone acetylations. FTY720 is also phosphorylated in mice and accumulates in the brain, including the hippocampus, inhibits HDACs and enhances histone acetylation and gene expression programs associated with memory and learning, and rescues memory deficits independently of its immunosuppressive actions. Sphk2(-/-) mice have lower levels of hippocampal sphingosine-1-phosphate, an endogenous HDAC inhibitor, and reduced histone acetylation, and display deficits in spatial memory and impaired contextual fear extinction. Thus, sphingosine-1-phosphate and SphK2 play specific roles in memory functions and FTY720 may be a useful adjuvant therapy to facilitate extinction of aversive memories. | Western Blotting | Mouse | 24859201
 |
Epstein-Barr virus-mediated transformation of B cells induces global chromatin changes independent to the acquisition of proliferation.
Hernando, H; Islam, AB; Rodríguez-Ubreva, J; Forné, I; Ciudad, L; Imhof, A; Shannon-Lowe, C; Ballestar, E
Nucleic acids research
42
249-63
2014
Show Abstract
Epstein-Barr virus (EBV) infects and transforms human primary B cells inducing indefinite proliferation. To investigate the potential participation of chromatin mechanisms during the EBV-mediated transformation of resting B cells we performed an analysis of global changes in histone modifications. We observed a remarkable decrease and redistribution of heterochromatin marks including H4K20me3, H3K27me3 and H3K9me3. Loss of H4K20me3 and H3K9me3 occurred at constitutive heterochromatin repeats. For H3K27me3 and H3K9me3, comparison of ChIP-seq data revealed a decrease in these marks in thousands of genes, including clusters of HOX and ZNF genes, respectively. Moreover, DNase-seq data comparison between resting and EBV-transformed B cells revealed increased endonuclease accessibility in thousands of genomic sites. We observed that both loss of H3K27me3 and increased accessibility are associated with transcriptional activation. These changes only occurred in B cells transformed with EBV and not in those stimulated to proliferate with CD40L/IL-4, despite their similarities in the cell pathways involved and proliferation rates. In fact, B cells infected with EBNA-2 deficient EBV, which have much lower proliferation rates, displayed similar decreases for heterochromatic histone marks. Our study describes a novel phenomenon related to transformation of B cells, and highlights its independence of the pure acquisition of proliferation. | Western Blotting | | 24097438
 |
The chromatin landscape of Drosophila: comparisons between species, sexes, and chromosomes.
Brown, EJ; Bachtrog, D
Genome research
24
1125-37
2014
Show Abstract
The chromatin landscape is key for gene regulation, but little is known about how it differs between sexes or between species. Here, we study the sex-specific chromatin landscape of Drosophila miranda, a species with young sex chromosomes, and compare it with Drosophila melanogaster. We analyze six histone modifications in male and female larvae of D. miranda (H3K4me1, H3K4me3, H3K36me3, H4K16ac, H3K27me3, and H3K9me2), and define seven biologically meaningful chromatin states that show different enrichments for transcribed and silent genes, repetitive elements, housekeeping, and tissue-specific genes. The genome-wide distribution of both active and repressive chromatin states differs between males and females. In males, active chromatin is enriched on the X, relative to females, due to dosage compensation of the hemizygous X. Furthermore, a smaller fraction of the euchromatic portion of the genome is in a repressive chromatin state in males relative to females. However, sex-specific chromatin states appear not to explain sex-biased expression of genes. Overall, conservation of chromatin states between male and female D. miranda is comparable to conservation between D. miranda and D. melanogaster, which diverged greater than 30 MY ago. Active chromatin states are more highly conserved across species, while heterochromatin shows very low levels of conservation. Divergence in chromatin profiles contributes to expression divergence between species, with ∼26% of genes in different chromatin states in the two species showing species-specific or species-biased expression, an enrichment of approximately threefold over null expectation. Our data suggest that heteromorphic sex chromosomes in males (that is, a hypertranscribed X and an inactivated Y) may contribute to global redistribution of active and repressive chromatin marks between chromosomes and sexes. | | | 24840603
 |
MOF-associated complexes ensure stem cell identity and Xist repression.
Chelmicki, T; Dündar, F; Turley, MJ; Khanam, T; Aktas, T; Ramírez, F; Gendrel, AV; Wright, PR; Videm, P; Backofen, R; Heard, E; Manke, T; Akhtar, A
eLife
3
e02024
2014
Show Abstract
Histone acetyl transferases (HATs) play distinct roles in many cellular processes and are frequently misregulated in cancers. Here, we study the regulatory potential of MYST1-(MOF)-containing MSL and NSL complexes in mouse embryonic stem cells (ESCs) and neuronal progenitors. We find that both complexes influence transcription by targeting promoters and TSS-distal enhancers. In contrast to flies, the MSL complex is not exclusively enriched on the X chromosome, yet it is crucial for mammalian X chromosome regulation as it specifically regulates Tsix, the major repressor of Xist lncRNA. MSL depletion leads to decreased Tsix expression, reduced REX1 recruitment, and consequently, enhanced accumulation of Xist and variable numbers of inactivated X chromosomes during early differentiation. The NSL complex provides additional, Tsix-independent repression of Xist by maintaining pluripotency. MSL and NSL complexes therefore act synergistically by using distinct pathways to ensure a fail-safe mechanism for the repression of X inactivation in ESCs.DOI: http://dx.doi.org/10.7554/eLife.02024.001. | | | 24842875
 |
Mof-associated complexes have overlapping and unique roles in regulating pluripotency in embryonic stem cells and during differentiation.
Ravens, S; Fournier, M; Ye, T; Stierle, M; Dembele, D; Chavant, V; Tora, L
eLife
3
2014
Show Abstract
The histone acetyltransferase (HAT) Mof is essential for mouse embryonic stem cell (mESC) pluripotency and early development. Mof is the enzymatic subunit of two different HAT complexes, MSL and NSL. The individual contribution of MSL and NSL to transcription regulation in mESCs is not well understood. Our genome-wide analysis show that i) MSL and NSL bind to specific and common sets of expressed genes, ii) NSL binds exclusively at promoters, iii) while MSL binds in gene bodies. Nsl1 regulates proliferation and cellular homeostasis of mESCs. MSL is the main HAT acetylating H4K16 in mESCs, is enriched at many mESC-specific and bivalent genes. MSL is important to keep a subset of bivalent genes silent in mESCs, while developmental genes require MSL for expression during differentiation. Thus, NSL and MSL HAT complexes differentially regulate specific sets of expressed genes in mESCs and during differentiation. | Western Blotting | | 24898753
 |
















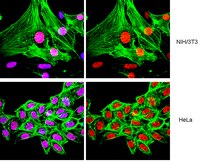
[07-329_ICC Acetyl-Histone H4 (Lys16)-ALL].jpg)
[07-329_ICC Acetyl-Histone H4 (Lys16)-ALL].jpg)

[07-329_DB Acetyl-Histone H4 (Lys16)-ALL].jpg)
[07-329_WB Acetyl-Histone H4 (Lys16)-ALL].jpg)