Modeling tau polymerization in vitro: a review and synthesis.
Gamblin, T Chris, et al.
Biochemistry, 42: 15009-17 (2003)
2003
Show Abstract
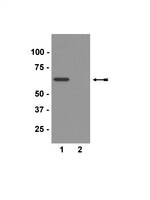
The major antigenic component of neurofibrillary pathology in a large number of neurodegenerative diseases consists of the microtubule-associated protein tau. It is currently unclear how tau protein makes the transition from an important component of the microtubule-based cytoskeleton to an insoluble polymerized state. In vitro techniques have been employed to study the polymerization of tau in an effort to understand the underlying molecular mechanisms responsible for this process. These efforts have resulted in the elucidation of roles played by the different parts of the molecule in the polymerization process. Here we discuss the advantages and disadvantages of the various techniques used to model tau polymerization and the discoveries arising from these techniques that have led to a better structural understanding of tau polymerization in relation to Alzheimer's disease and other tauopathies. | 14690409
 |
The slow axonal transport of the microtubule-associated protein tau and the transport rates of different isoforms and mutants in cultured neurons.
Utton, Michelle A, et al.
J. Neurosci., 22: 6394-400 (2002)
2002
Show Abstract
We demonstrate that the microtubule-associated protein tau, in the form of enhanced green fluorescent protein (EGFP) tau, is transported along axons of neurons in culture in the slow component of axonal transport with a speed comparable with that previously measured in vivo. It was demonstrated that the EGFP tag has no effect on transport characteristics, and the methodology enables slow transport rates of individual tau isoforms and tau mutants to be measured. We also expressed EGFP-tagged tau isoforms containing either three or four C-terminal repeats and zero or two N-terminal inserts in cultured neurons. No significant differences were found in the average rate of slow transport of the wild-type tau isoforms, suggesting that the exon 10 C-terminal repeat or the N-terminal inserts do not contain regions that play a significant regulatory role in axonal transport. Similarly, we found that missense mutations in tau have no noticeable effect on the rate of transport; hence their ability to cause neurodegeneration is by another mechanism other than that affecting the overall slow axonal transport of tau. | 12151518
 |