Progesterone receptor enhances breast cancer cell motility and invasion via extranuclear activation of focal adhesion kinase.
Fu, XD; Goglia, L; Sanchez, AM; Flamini, M; Giretti, MS; Tosi, V; Genazzani, AR; Simoncini, T
Endocrine-related cancer
17
431-43
2010
Show Abstract
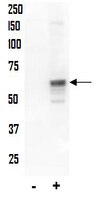
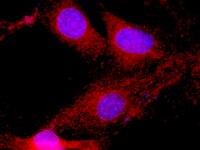
While progesterone plays multiple roles in the process of breast development and differentiation, its role in breast cancer is less understood. We have shown previously that progestins stimulate breast cancer cell migration and invasion because of the activation of rapid signaling cascades leading to modifications in the actin cytoskeleton and cell membrane that are required for cell movement. In this study, we have investigated the effects of progesterone on the formation of focal adhesion (FA) complexes, which provide anchoring sites for cell attachment to the extracellular matrix during cell movement and invasion. In T47-D breast cancer cells, progesterone rapidly enhances FA kinase (FAK) phosphorylation at Tyr(397) in a time- and concentration-dependent manner. As a result, exposure to progesterone leads to increased formation of FA complexes within specialized cell membrane protrusions. The cascade of events required for this phenomenon involves progesterone receptor interaction with the tyrosine kinase c-Src, which activates the phosphatidylinositol-3-kinase/Akt pathway and the small GTPase RhoA/Rho-associated kinase complex. In the presence of progesterone, T47-D breast cancer cells display enhanced horizontal migration and invasion of three-dimensional matrices, which is reversed by small interfering RNAs abrogating FAK. In conclusion, progesterone promotes breast cancer cell movement and invasion by facilitating the formation of FA complexes via the rapid regulation of FAK. These results provide novel mechanistic views on the effects of progesterone on breast cancer progression, and may in the future be helpful to develop new strategies for the treatment of endocrine-sensitive breast cancers. | Western Blotting | 20233709
 |
Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton.
Fu, XD; Giretti, MS; Baldacci, C; Garibaldi, S; Flamini, M; Sanchez, AM; Gadducci, A; Genazzani, AR; Simoncini, T
PloS one
3
e2790
2008
Show Abstract
Progesterone plays a role in breast cancer development and progression but the effects on breast cancer cell movement or invasion have not been fully explored. In this study, we investigate the actions of natural progesterone and of the synthetic progestin medroxyprogesterone acetate (MPA) on actin cytoskeleton remodeling and on breast cancer cell movement and invasion. In particular, we characterize the nongenomic signaling cascades implicated in these actions. T47-D breast cancer cells display enhanced horizontal migration and invasion of three-dimensional matrices in the presence of both progestins. Exposure to the hormones triggers a rapid remodeling of the actin cytoskeleton and the formation of membrane ruffles required for cell movement, which are dependent on the rapid phosphorylation of the actin-regulatory protein moesin. The extra-cellular small GTPase RhoA/Rho-associated kinase (ROCK-2) cascade plays central role in progesterone- and MPA-induced moesin activation, cell migration and invasion. In the presence of progesterone, progesterone receptor A (PRA) interacts with the G protein G alpha(13), while MPA drives PR to interact with tyrosine kinase c-Src and to activate phosphatidylinositol-3 kinase, leading to the activation of RhoA/ROCK-2. In conclusion, our findings manifest that progesterone and MPA promote breast cancer cell movement via rapid actin cytoskeleton remodeling, which are mediated by moesin activation. These events are triggered by RhoA/ROCK-2 cascade through partially differing pathways by the two compounds. These results provide original mechanistic explanations for the effects of progestins on breast cancer progression and highlight potential targets to treat endocrine-sensitive breast cancers. Full Text Article | Immunoblotting (Western) | 18665217
 |
PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond.
Marte, B M and Downward, J
Trends Biochem. Sci., 22: 355-8 (1997)
1997
Show Abstract
PKB/Akt is a serine/threonine kinase that contains a pleckstrin-homology (PH) domain and is activated in response to growth-factor treatment of cells by a mechanism involving phosphoinositide 3-OH kinase. PKB/Akt provides a survival signal that protects cells from apoptosis induced by various stresses, perhaps explaining its discovery as a retroviral oncogene and its amplification in many human tumours. | | 9301337
 |