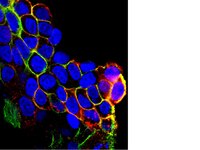
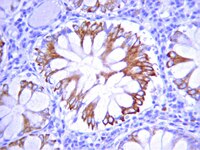
TIMP-1 attenuates blood-brain barrier permeability in mice with acute liver failure.
Chen, F; Radisky, ES; Das, P; Batra, J; Hata, T; Hori, T; Baine, AM; Gardner, L; Yue, MY; Bu, G; del Zoppo, G; Patel, TC; Nguyen, JH
Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism
33
1041-9
2013
Show Abstract
Blood-brain barrier (BBB) dysfunction in acute liver failure (ALF) results in increased BBB permeability that often precludes the patients from obtaining a life-saving liver transplantation. It remains controversial whether matrix metalloproteinase-9 (MMP-9) from the injured liver contributes to the deregulation of BBB function in ALF. We selectively upregulated a physiologic inhibitor of MMP-9 (TIMP-1) with a single intracerebroventricular injection of TIMP-1 cDNA plasmids at 48 and 72 hours, or with pegylated-TIMP-1 protein. Acute liver failure was induced with tumor necrosis factor-α and D-(+)-galactosamine in mice. Permeability of BBB was assessed with sodium fluorescein (NaF) extravasation. We found a significant increase in TIMP-1 within the central nervous system (CNS) after the administration of TIMP-1 cDNA plasmids and that increased TIMP-1 within the CNS resulted in an attenuation of BBB permeability, a reduction in activation of epidermal growth factor receptor and p38 mitogen-activated protein kinase signals, and a restoration of the tight junction protein occludin in mice with experimental ALF. Pegylated TIMP-1 provided similar protection against BBB permeability in mice with ALF. Our results provided a proof of principle that MMP-9 contributes to the BBB dysfunction in ALF and suggests a potential therapeutic role of TIMP-1 in ALF. | Western Blotting | Mouse | 23532086
 |
Immunodetection of the ligand-activated receptor for epidermal growth factor.
Campos-González, R and Glenney, J R
Growth Factors, 4: 305-16 (1991)
1991
Show Abstract
Many receptors for cellular growth factors are known to be protein tyrosine kinases which become activated upon ligand binding at their extracellular domain. We describe here a method to detect the activation state of Epidermal Growth Factor receptor (EGFr) with a monoclonal antibody (mAb74). This antibody was found to preferentially recognize the ligand-activated EGFr as detected by immunoprecipitation, Western blotting and immunocytochemical techniques. mAb74 did not recognize other tyrosine-phosphorylated proteins and was not inhibited by phosphotyrosine, suggesting that it is recognizing an epitope specific for the ligand-activated EGF receptor. The reactivity of mAb74 towards EGFr was closely correlated with the EGF-dependent tyrosine phosphorylation of endogenous substrates. This antibody allows one to detect the activated EGF receptor in vitro or in vivo even in a complex mixture of other tyrosine kinases and substrates. | | | 1722417
 |