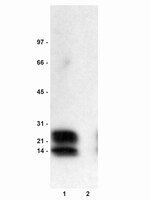
Atrial fibrillation complicated by heart failure induces distinct remodeling of calcium cycling proteins.
Lugenbiel, P; Wenz, F; Govorov, K; Schweizer, PA; Katus, HA; Thomas, D
PloS one
10
e0116395
2015
Show Abstract
Atrial fibrillation (AF) and heart failure (HF) are two of the most common cardiovascular diseases. They often coexist and account for significant morbidity and mortality. Alterations in cellular Ca2+ homeostasis play a critical role in AF initiation and maintenance. This study was designed to specifically elucidate AF-associated remodeling of atrial Ca2+ cycling in the presence of mild HF. AF was induced in domestic pigs by atrial burst pacing. The animals underwent electrophysiologic and echocardiographic examinations. Ca2+ handling proteins were analyzed in right atrial tissue obtained from pigs with AF (day 7; n = 5) and compared to sinus rhythm (SR) controls (n = 5). During AF, animals exhibited reduction of left ventricular ejection fraction (from 73% to 58%) and prolonged atrial refractory periods. AF and HF were associated with suppression of protein kinase A (PKA)RII (-62%) and Ca2+-calmodulin-dependent kinase II (CaMKII) δ by 37%, without changes in CaMKIIδ autophosphorylation. We further detected downregulation of L-type calcium channel (LTCC) subunit α2 (-75%), sarcoplasmic reticulum Ca2+-ATPase (Serca) 2a (-29%), phosphorylated phospholamban (Ser16, -92%; Thr17, -70%), and phospho-ryanodine receptor 2 (RyR2) (Ser2808, -62%). Na+-Ca2+ exchanger (NCX) levels were upregulated (+473%), whereas expression of Ser2814-phosphorylated RyR2 and LTCCα1c subunits was not significantly altered. In conclusion, AF produced distinct arrhythmogenic remodeling of Ca2+ handling in the presence of tachycardia-induced mild HF that is different from AF without structural alterations. The changes may provide a starting point for personalized approaches to AF treatment. | | 25775120
 |
Pak1 is required to maintain ventricular Ca²⁺ homeostasis and electrophysiological stability through SERCA2a regulation in mice.
Wang, Y; Tsui, H; Ke, Y; Shi, Y; Li, Y; Davies, L; Cartwright, EJ; Venetucci, L; Zhang, H; Terrar, DA; Huang, CL; Solaro, RJ; Wang, X; Lei, M
Circulation. Arrhythmia and electrophysiology
7
938-48
2014
Show Abstract
Impaired sarcoplasmic reticular Ca(2+) uptake resulting from decreased sarcoplasmic reticulum Ca(2+)-ATPase type 2a (SERCA2a) expression or activity is a characteristic of heart failure with its associated ventricular arrhythmias. Recent attempts at gene therapy of these conditions explored strategies enhancing SERCA2a expression and the activity as novel approaches to heart failure management. We here explore the role of Pak1 in maintaining ventricular Ca(2+) homeostasis and electrophysiological stability under both normal physiological and acute and chronic β-adrenergic stress conditions.Mice with a cardiomyocyte-specific Pak1 deletion (Pak1(cko)), but not controls (Pak1(f/f)), showed high incidences of ventricular arrhythmias and electrophysiological instability during either acute β-adrenergic or chronic β-adrenergic stress leading to hypertrophy, induced by isoproterenol. Isolated Pak1(cko) ventricular myocytes correspondingly showed aberrant cellular Ca(2+) homeostasis. Pak1(cko) hearts showed an associated impairment of SERCA2a function and downregulation of SERCA2a mRNA and protein expression. Further explorations of the mechanisms underlying the altered transcriptional regulation demonstrated that exposure to control Ad-shC2 virus infection increased SERCA2a protein and mRNA levels after phenylephrine stress in cultured neonatal rat cardiomyocytes. This was abolished by the Pak1-knockdown in Ad-shPak1-infected neonatal rat cardiomyocytes and increased by constitutive overexpression of active Pak1 (Ad-CAPak1). We then implicated activation of serum response factor, a transcriptional factor well known for its vital role in the regulation of cardiogenesis genes in the Pak1-dependent regulation of SERCA2a.These findings indicate that Pak1 is required to maintain ventricular Ca(2+) homeostasis and electrophysiological stability and implicate Pak1 as a novel regulator of cardiac SERCA2a through a transcriptional mechanism. | | 25217043
 |
Long term ablation of protein kinase A (PKA)-mediated cardiac troponin I phosphorylation leads to excitation-contraction uncoupling and diastolic dysfunction in a knock-in mouse model of hypertrophic cardiomyopathy.
Dweck, D; Sanchez-Gonzalez, MA; Chang, AN; Dulce, RA; Badger, CD; Koutnik, AP; Ruiz, EL; Griffin, B; Liang, J; Kabbaj, M; Fincham, FD; Hare, JM; Overton, JM; Pinto, JR
The Journal of biological chemistry
289
23097-111
2014
Show Abstract
The cardiac troponin I (cTnI) R21C (cTnI-R21C) mutation has been linked to hypertrophic cardiomyopathy and renders cTnI incapable of phosphorylation by PKA in vivo. Echocardiographic imaging of homozygous knock-in mice expressing the cTnI-R21C mutation shows that they develop hypertrophy after 12 months of age and have abnormal diastolic function that is characterized by longer filling times and impaired relaxation. Electrocardiographic analyses show that older R21C mice have elevated heart rates and reduced cardiovagal tone. Cardiac myocytes isolated from older R21C mice demonstrate that in the presence of isoproterenol, significant delays in Ca(2+) decay and sarcomere relaxation occur that are not present at 6 months of age. Although isoproterenol and stepwise increases in stimulation frequency accelerate Ca(2+)-transient and sarcomere shortening kinetics in R21C myocytes from older mice, they are unable to attain the corresponding WT values. When R21C myocytes from older mice are treated with isoproterenol, evidence of excitation-contraction uncoupling is indicated by an elevation in diastolic calcium that is frequency-dissociated and not coupled to shorter diastolic sarcomere lengths. Myocytes from older mice have smaller Ca(2+) transient amplitudes (2.3-fold) that are associated with reductions (2.9-fold) in sarcoplasmic reticulum Ca(2+) content. This abnormal Ca(2+) handling within the cell may be attributed to a reduction (2.4-fold) in calsequestrin expression in conjunction with an up-regulation (1.5-fold) of Na(+)-Ca(2+) exchanger. Incubation of permeabilized cardiac fibers from R21C mice with PKA confirmed that the mutation prevents facilitation of mechanical relaxation. Altogether, these results indicate that the inability to enhance myofilament relaxation through cTnI phosphorylation predisposes the heart to abnormal diastolic function, reduced accessibility of cardiac reserves, dysautonomia, and hypertrophy. | Western Blotting | 24973218
 |
Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice.
Patel, BB; Raad, M; Sebag, IA; Chalifour, LE
Toxicological sciences : an official journal of the Society of Toxicology
133
174-85
2013
Show Abstract
Bisphenol A (BPA) is an estrogenizing endocrine disruptor compound of concern. Our objective was to test whether lifelong BPA would impact cardiac structure/function, calcium homeostasis protein expression, and the DNA methylation of cardiac genes. We delivered 0.5 and 5.0 µg/kg/day BPA lifelong from gestation day 11 or 200 µg/kg/day from gestation day 11 to postnatal day 21 via the drinking water to C57bl/6n mice. BPA 5.0 males and females had increased body weight, body mass index, body surface area, and adiposity. Echocardiography identified concentric remodeling in all BPA-treated males. Systolic and diastolic cardiac functions were essentially similar, but lifelong BPA enhanced male and reduced female sex-specific differences in velocity of circumferential shortening and ascending aorta velocity time integral. Diastolic blood pressure was increased in all BPA females. The calcium homeostasis proteins sarcoendoplasmic reticulum ATPase 2a (SERCA2a), sodium calcium exchanger-1, phospholamban (PLB), phospho-PLB, and calsequestrin 2 are important for contraction and relaxation. Changes in their expression suggest increased calcium mobility in males and reduced calcium mobility in females supporting the cardiac function changes. DNA methyltransferase 3a expression was increased in all BPA males and BPA 0.5 females and reduced in BPA 200 females. Global DNA methylation was increased in BPA 0.5 males and reduced in BPA 0.5 females. BPA induced sex-specific altered DNA methylation in specific CpG pairs in the calsequestrin 2 CpG island. These results suggest that continual exposure to BPA impacts cardiac structure/function, protein expression, and epigenetic DNA methylation marks in males and females. | | 23418087
 |
Anthrax lethal toxin induces acute diastolic dysfunction in rats through disruption of the phospholamban signaling network.
Golden, HB; Watson, LE; Nizamutdinov, D; Feng, H; Gerilechaogetu, F; Lal, H; Verma, SK; Mukhopadhyay, S; Foster, DM; Dillmann, WH; Dostal, DE
International journal of cardiology
168
3884-95
2013
Show Abstract
Anthrax lethal toxin (LT), secreted by Bacillus anthracis, causes severe cardiac dysfunction by unknown mechanisms. LT specifically cleaves the docking domains of MAPKK (MEKs); thus, we hypothesized that LT directly impairs cardiac function through dysregulation of MAPK signaling mechanisms.In a time-course study of LT toxicity, echocardiography revealed acute diastolic heart failure accompanied by pulmonary regurgitation and left atrial dilation in adult Sprague-Dawley rats at time points corresponding to dysregulated JNK, phospholamban (PLB) and protein phosphatase 2A (PP2A) myocardial signaling. Using isolated rat ventricular myocytes, we identified the MEK7-JNK1-PP2A-PLB signaling axis to be important for regulation of intracellular calcium (Ca(2+)(i)) handling, PP2A activation and targeting of PP2A-B56α to Ca(2+)(i) handling proteins, such as PLB. Through a combination of gain-of-function and loss-of-function studies, we demonstrated that over-expression of MEK7 protects against LT-induced PP2A activation and Ca(2+)(i) dysregulation through activation of JNK1. Moreover, targeted phosphorylation of PLB-Thr(17) by Akt improved sarcoplasmic reticulum Ca(2+)(i) release and reuptake during LT toxicity. Co-immunoprecipitation experiments further revealed the pivotal role of MEK7-JNK-Akt complex formation for phosphorylation of PLB-Thr(17) during acute LT toxicity.Our findings support a cardiogenic mechanism of LT-induced diastolic dysfunction, by which LT disrupts JNK1 signaling and results in Ca(2+)(i) dysregulation through diminished phosphorylation of PLB by Akt and increased dephosphorylation of PLB by PP2A. Integration of the MEK7-JNK1 signaling module with Akt represents an important stress-activated signalosome that may confer protection to sustain cardiac contractility and maintain normal levels of Ca(2+)(i) through PLB-T(17) phosphorylation. | | 23907041
 |
Noncanonical EF-hand motif strategically delays Ca2+ buffering to enhance cardiac performance.
Wang, W; Barnabei, MS; Asp, ML; Heinis, FI; Arden, E; Davis, J; Braunlin, E; Li, Q; Davis, JP; Potter, JD; Metzger, JM
Nature medicine
19
305-12
2013
Show Abstract
EF-hand proteins are ubiquitous in cell signaling. Parvalbumin (Parv), the archetypal EF-hand protein, is a high-affinity Ca(2+) buffer in many biological systems. Given the centrality of Ca(2+) signaling in health and disease, EF-hand motifs designed to have new biological activities may have widespread utility. Here, an EF-hand motif substitution that had been presumed to destroy EF-hand function, that of glutamine for glutamate at position 12 of the second cation binding loop domain of Parv (ParvE101Q), markedly inverted relative cation affinities: Mg(2+) affinity increased, whereas Ca(2+) affinity decreased, forming a new ultra-delayed Ca(2+) buffer with favorable properties for promoting cardiac relaxation. In therapeutic testing, expression of ParvE101Q fully reversed the severe myocyte intrinsic contractile defect inherent to expression of native Parv and corrected abnormal myocardial relaxation in diastolic dysfunction disease models in vitro and in vivo. Strategic design of new EF-hand motif domains to modulate intracellular Ca(2+) signaling could benefit many biological systems with abnormal Ca(2+) handling, including the diseased heart. | | 23396207
 |
Impaired contractile function due to decreased cardiac myosin binding protein C content in the sarcomere.
Cheng, Y; Wan, X; McElfresh, TA; Chen, X; Gresham, KS; Rosenbaum, DS; Chandler, MP; Stelzer, JE
American journal of physiology. Heart and circulatory physiology
305
H52-65
2013
Show Abstract
Mutations in cardiac myosin binding protein C (MyBP-C) are a common cause of familial hypertrophic cardiomyopathy (FHC). The majority of MyBP-C mutations are expected to reduce MyBP-C expression; however, the consequences of MyBP-C deficiency on the regulation of myofilament function, Ca²⁺ homeostasis, and in vivo cardiac function are unknown. To elucidate the effects of decreased MyBP-C expression on cardiac function, we employed MyBP-C heterozygous null (MyBP-C+/-) mice presenting decreases in MyBP-C expression (32%) similar to those of FHC patients carrying MyBP-C mutations. The levels of MyBP-C phosphorylation were reduced 53% in MyBP-C+/- hearts compared with wild-type hearts. Skinned myocardium isolated from MyBP-C+/- hearts displayed decreased cross-bridge stiffness at half-maximal Ca²⁺ activations, increased steady-state force generation, and accelerated rates of cross-bridge recruitment at low Ca²⁺ activations (less than 15% and less than 25% of maximum, respectively). Protein kinase A treatment abolished basal differences in rates of cross-bridge recruitment between MyBP-C+/- and wild-type myocardium. Intact ventricular myocytes from MyBP-C+/- hearts displayed abnormal sarcomere shortening but unchanged Ca²⁺ transient kinetics. Despite a lack of left ventricular hypertrophy, MyBP-C+/- hearts exhibited elevated end-diastolic pressure and decreased peak rate of LV pressure rise, which was normalized following dobutamine infusion. Furthermore, electrocardiogram recordings in conscious MyBP-C+/- mice revealed prolonged QRS and QT intervals, which are known risk factors for cardiac arrhythmia. Collectively, our data show that reduced MyBP-C expression and phosphorylation in the sarcomere result in myofilament dysfunction, contributing to contractile dysfunction that precedes compensatory adaptations in Ca²⁺ handling, and chamber remodeling. Perturbations in mechanical and electrical activity in MyBP-C+/- mice could increase their susceptibility to cardiac dysfunction and arrhythmia. | | 23666674
 |
The effects of neuregulin on cardiac Myosin light chain kinase gene-ablated hearts.
Chang, AN; Huang, J; Battiprolu, PK; Hill, JA; Kamm, KE; Stull, JT
PloS one
8
e66720
2013
Show Abstract
Activation of ErbB2/4 receptor tyrosine kinases in cardiomyocytes by neuregulin treatment is associated with improvement in cardiac function, supporting its use in human patients with heart failure despite the lack of a specific mechanism. Neuregulin infusion in rodents increases cardiac myosin light chain kinase (cMLCK) expression and cardiac myosin regulatory light chain (RLC) phosphorylation which may improve actin-myosin interactions for contraction. We generated a cMLCK knockout mouse to test the hypothesis that cMLCK is necessary for neuregulin-induced improvement in cardiac function by increasing RLC phosphorylation.The cMLCK knockout mice have attenuated RLC phosphorylation and decreased cardiac performance measured as fractional shortening. Neuregulin infusion for seven days in wildtype mice increased cardiac cMLCK protein expression and RLC phosphorylation while increasing Akt phosphorylation and decreasing phospholamban phosphorylation. There was no change in fractional shortening. In contrast, neuregulin infusion in cMLCK knockout animals increased cardiac performance in the absence of cMLCK without increasing RLC phosphorylation. In addition, CaMKII signaling appeared to be enhanced in neuregulin-treated knockout mice.Thus, Neuregulin may improve cardiac performance in the failing heart without increasing cMLCK and RLC phosphorylation by activating other signaling pathways. | | 23776695
 |
SERCA Cys674 sulphonylation and inhibition of L-type Ca2+ influx contribute to cardiac dysfunction in endotoxemic mice, independent of cGMP synthesis.
Hobai, IA; Buys, ES; Morse, JC; Edgecomb, J; Weiss, EH; Armoundas, AA; Hou, X; Khandelwal, AR; Siwik, DA; Brouckaert, P; Cohen, RA; Colucci, WS
American journal of physiology. Heart and circulatory physiology
305
H1189-200
2013
Show Abstract
The goal of this study was to identify the cellular mechanisms responsible for cardiac dysfunction in endotoxemic mice. We aimed to differentiate the roles of cGMP [produced by soluble guanylyl cyclase (sGC)] versus oxidative posttranslational modifications of Ca(2+) transporters. C57BL/6 mice [wild-type (WT) mice] were administered lipopolysaccharide (LPS; 25 μg/g ip) and euthanized 12 h later. Cardiomyocyte sarcomere shortening and Ca(2+) transients (ΔCai) were depressed in LPS-challenged mice versus baseline. The time constant of Ca(2+) decay (τCa) was prolonged, and sarcoplasmic reticulum Ca(2+) load (CaSR) was depressed in LPS-challenged mice (vs. baseline), indicating decreased activity of sarco(endo)plasmic Ca(2+)-ATPase (SERCA). L-type Ca(2+) channel current (ICa,L) was also decreased after LPS challenge, whereas Na(+)/Ca(2+) exchange activity, ryanodine receptors leak flux, or myofilament sensitivity for Ca(2+) were unchanged. All Ca(2+)-handling abnormalities induced by LPS (the decrease in sarcomere shortening, ΔCai, CaSR, ICa,L, and τCa prolongation) were more pronounced in mice deficient in the sGC main isoform (sGCα1(-/-) mice) versus WT mice. LPS did not alter the protein expression of SERCA and phospholamban in either genotype. After LPS, phospholamban phosphorylation at Ser(16) and Thr(17) was unchanged in WT mice and was increased in sGCα1(-/-) mice. LPS caused sulphonylation of SERCA Cys(674) (as measured immunohistochemically and supported by iodoacetamide labeling), which was greater in sGCα1(-/-) versus WT mice. Taken together, these results suggest that cardiac Ca(2+) dysregulation in endotoxemic mice is mediated by a decrease in L-type Ca(2+) channel function and oxidative posttranslational modifications of SERCA Cys(674), with the latter (at least) being opposed by sGC-released cGMP. | | 23934853
 |
Cardiac remodeling and myocardial dysfunction in obese spontaneously hypertensive rats.
Linz, D; Hohl, M; Mahfoud, F; Reil, JC; Linz, W; Hübschle, T; Juretschke, HP; Neumann-Häflin, C; Rütten, H; Böhm, M
Journal of translational medicine
10
187
2012
Show Abstract
The additive effects of obesity and metabolic syndrome on left ventricular (LV) maladaptive remodeling and function in hypertension are not characterized.We compared an obese spontaneously hypertensive rat model (SHR-ob) with lean spontaneously hypertensive rats (SHR-lean) and normotensive controls (Ctr). LV-function was investigated by cardiac magnetic resonance imaging and invasive LV-pressure measurements. LV-interstitial fibrosis was quantified and protein levels of phospholamban (PLB), Serca2a and glucose transporters (GLUT1 and GLUT4) were determined by immunohistochemistry.Systolic blood pressure was similar in SHR-lean and SHR-ob (252 ± 7 vs. 242 ± 7 mmHg, p = 0.398) but was higher when compared to Ctr (155 ± 2 mmHg, p less than 0.01 for both). Compared to SHR-lean and Ctr, SHR-ob showed impaired glucose tolerance and increased body-weight. In SHR-ob, LV-ejection fraction was impaired vs. Ctr (46.2 ± 1.1 vs. 59.6 ± 1.9%, p = 0.007). LV-enddiastolic pressure was more increased in SHR-ob than in SHR-lean (21.5 ± 4.1 vs. 5.9 ± 0.81 mmHg, p = 0.0002) when compared to Ctr (4.3 ± 1.1 mmHg, p less than 0.0001 for both), respectively. Increased LV-fibrosis together with increased myocyte diameters and ANF gene expression in SHR-ob were associated with increased GLUT1-protein levels in SHR-ob suggestive for an upregulation of the GLUT1/ANF-axis. Serca2a-protein levels were decreased in SHR-lean but not altered in SHR-ob compared to Ctr. PLB-phosphorylation was not altered.In addition to hypertension alone, metabolic syndrome and obesity adds to the myocardial phenotype by aggravating diastolic dysfunction and a progression towards systolic dysfunction. SHR-ob may be a useful model to develop new interventional and pharmacological treatment strategies for hypertensive heart disease and metabolic disorders. | Western Blotting | 22963383
 |