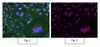
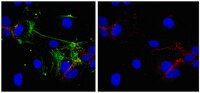
Maternal anti-platelet β3 integrins impair angiogenesis and cause intracranial hemorrhage.
Yougbaré, I; Lang, S; Yang, H; Chen, P; Zhao, X; Tai, WS; Zdravic, D; Vadasz, B; Li, C; Piran, S; Marshall, A; Zhu, G; Tiller, H; Killie, MK; Boyd, S; Leong-Poi, H; Wen, XY; Skogen, B; Adamson, SL; Freedman, J; Ni, H
The Journal of clinical investigation
125
1545-56
2015
Show Abstract
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is a life-threatening disease in which intracranial hemorrhage (ICH) is the major risk. Although thrombocytopenia, which is caused by maternal antibodies against β3 integrin and occasionally by maternal antibodies against other platelet antigens, such as glycoprotein GPIbα, has long been assumed to be the cause of bleeding, the mechanism of ICH has not been adequately explored. Utilizing murine models of FNAIT and a high-frequency ultrasound imaging system, we found that ICH only occurred in fetuses and neonates with anti-β3 integrin-mediated, but not anti-GPIbα-mediated, FNAIT, despite similar thrombocytopenia in both groups. Only anti-β3 integrin-mediated FNAIT reduced brain and retina vessel density, impaired angiogenic signaling, and increased endothelial cell apoptosis, all of which were abrogated by maternal administration of intravenous immunoglobulin (IVIG). ICH and impairment of retinal angiogenesis were further reproduced in neonates by injection of anti-β3 integrin, but not anti-GPIbα antisera. Utilizing cultured human endothelial cells, we found that cell proliferation, network formation, and AKT phosphorylation were inhibited only by murine anti-β3 integrin antisera and human anti-HPA-1a IgG purified from mothers with FNAIT children. Our data suggest that fetal hemostasis is distinct and that impairment of angiogenesis rather than thrombocytopenia likely causes FNAIT-associated ICH. Additionally, our results indicate that maternal IVIG therapy can effectively prevent this devastating disorder. | | | 25774504
 |
Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis.
Noda, S; Asano, Y; Nishimura, S; Taniguchi, T; Fujiu, K; Manabe, I; Nakamura, K; Yamashita, T; Saigusa, R; Akamata, K; Takahashi, T; Ichimura, Y; Toyama, T; Tsuruta, D; Trojanowska, M; Nagai, R; Sato, S
Nature communications
5
5797
2014
Show Abstract
Systemic sclerosis (SSc) is manifested by fibrosis, vasculopathy and immune dysregulation. So far, a unifying hypothesis underpinning these pathological events remains unknown. Given that SSc is a multifactorial disease caused by both genetic and environmental factors, we focus on the two transcription factors, which modulate the fibrotic reaction and are epigenetically suppressed in SSc dermal fibroblasts, Friend leukaemia integration 1 (Fli1) and Krüppel-like factor 5 (KLF5). In addition to the Fli1 silencing-dependent collagen induction, the simultaneous knockdown of Fli1 and KLF5 synergistically enhances expression of connective tissue growth factor. Notably, mice with double heterozygous deficiency of Klf5 and Fli1 mimicking the epigenetic phenotype of SSc skin spontaneously recapitulate all the three features of SSc, including fibrosis and vasculopathy of the skin and lung, B-cell activation and autoantibody production. These studies implicate the epigenetic downregulation of Fli1 and KLF5 as a central event triggering the pathogenic triad of SSc. | Immunohistochemistry | | 25504335
 |
A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone.
Thibaudeau, L; Taubenberger, AV; Holzapfel, BM; Quent, VM; Fuehrmann, T; Hesami, P; Brown, TD; Dalton, PD; Power, CA; Hollier, BG; Hutmacher, DW
Disease models & mechanisms
7
299-309
2014
Show Abstract
The skeleton is a preferred homing site for breast cancer metastasis. To date, treatment options for patients with bone metastases are mostly palliative and the disease is still incurable. Indeed, key mechanisms involved in breast cancer osteotropism are still only partially understood due to the lack of suitable animal models to mimic metastasis of human tumor cells to a human bone microenvironment. In the presented study, we investigate the use of a human tissue-engineered bone construct to develop a humanized xenograft model of breast cancer-induced bone metastasis in a murine host. Primary human osteoblastic cell-seeded melt electrospun scaffolds in combination with recombinant human bone morphogenetic protein 7 were implanted subcutaneously in non-obese diabetic/severe combined immunodeficient mice. The tissue-engineered constructs led to the formation of a morphologically intact 'organ' bone incorporating a high amount of mineralized tissue, live osteocytes and bone marrow spaces. The newly formed bone was largely humanized, as indicated by the incorporation of human bone cells and human-derived matrix proteins. After intracardiac injection, the dissemination of luciferase-expressing human breast cancer cell lines to the humanized bone ossicles was detected by bioluminescent imaging. Histological analysis revealed the presence of metastases with clear osteolysis in the newly formed bone. Thus, human tissue-engineered bone constructs can be applied efficiently as a target tissue for human breast cancer cells injected into the blood circulation and replicate the osteolytic phenotype associated with breast cancer-induced bone lesions. In conclusion, we have developed an appropriate model for investigation of species-specific mechanisms of human breast cancer-related bone metastasis in vivo. | | | 24713276
 |
Antagonizing the αv β3 integrin inhibits angiogenesis and impairs woven but not lamellar bone formation induced by mechanical loading.
Tomlinson, RE; Schmieder, AH; Quirk, JD; Lanza, GM; Silva, MJ
Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research
29
1970-80
2014
Show Abstract
Angiogenesis and osteogenesis are critically linked, although the role of angiogenesis is not well understood in osteogenic mechanical loading. In this study, either damaging or non-damaging cyclic axial compression was used to generate woven bone formation (WBF) or lamellar bone formation (LBF), respectively, at the mid-diaphysis of the adult rat forelimb. αv β3 integrin-targeted nanoparticles or vehicle was injected intravenously after mechanical loading. β3 integrin subunit expression on vasculature was maximal 7 days after damaging mechanical loading, but was still robustly expressed 14 days after loading. Accordingly, targeted nanoparticle delivery in WBF-loaded limbs was increased compared with non-loaded limbs. Vascularity was dramatically increased after WBF loading (+700% on day 14) and modestly increased after LBF loading (+50% on day 14). This increase in vascularity was inhibited by nanoparticle treatment in both WBF- and LBF-loaded limbs at days 7 and 14 after loading. Decreased vascularity led to diminished woven, but not lamellar, bone formation. Decreased woven bone formation resulted in impaired structural properties of the skeletal repair, particularly in post-yield behavior. These results demonstrate that αv β3 integrin-mediated angiogenesis is critical for recovering fracture resistance after bone injury but is not required for bone modeling after modest mechanical strain. © 2014 American Society for Bone and Mineral Research. | | | 24644077
 |
Multiphasic construct studied in an ectopic osteochondral defect model.
Jeon, JE; Vaquette, C; Theodoropoulos, C; Klein, TJ; Hutmacher, DW
Journal of the Royal Society, Interface / the Royal Society
11
20140184
2014
Show Abstract
In vivo osteochondral defect models predominantly consist of small animals, such as rabbits. Although they have an advantage of low cost and manageability, their joints are smaller and more easily healed compared with larger animals or humans. We hypothesized that osteochondral cores from large animals can be implanted subcutaneously in rats to create an ectopic osteochondral defect model for routine and high-throughput screening of multiphasic scaffold designs and/or tissue-engineered constructs (TECs). Bovine osteochondral plugs with 4 mm diameter osteochondral defect were fitted with novel multiphasic osteochondral grafts composed of chondrocyte-seeded alginate gels and osteoblast-seeded polycaprolactone scaffolds, prior to being implanted in rats subcutaneously with bone morphogenic protein-7. After 12 weeks of in vivo implantation, histological and micro-computed tomography analyses demonstrated that TECs are susceptible to mineralization. Additionally, there was limited bone formation in the scaffold. These results suggest that the current model requires optimization to facilitate robust bone regeneration and vascular infiltration into the defect site. Taken together, this study provides a proof-of-concept for a high-throughput osteochondral defect model. With further optimization, the presented hybrid in vivo model may address the growing need for a cost-effective way to screen osteochondral repair strategies before moving to large animal preclinical trials. | | | 24694896
 |
Multi-omic integrated networks connect DNA methylation and miRNA with skeletal muscle plasticity to chronic exercise in Type 2 diabetic obesity.
Rowlands, DS; Page, RA; Sukala, WR; Giri, M; Ghimbovschi, SD; Hayat, I; Cheema, BS; Lys, I; Leikis, M; Sheard, PW; Wakefield, SJ; Breier, B; Hathout, Y; Brown, K; Marathi, R; Orkunoglu-Suer, FE; Devaney, JM; Leiken, B; Many, G; Krebs, J; Hopkins, WG; Hoffman, EP
Physiological genomics
46
747-65
2014
Show Abstract
Epigenomic regulation of the transcriptome by DNA methylation and posttranscriptional gene silencing by miRNAs are potential environmental modulators of skeletal muscle plasticity to chronic exercise in healthy and diseased populations. We utilized transcriptome networks to connect exercise-induced differential methylation and miRNA with functional skeletal muscle plasticity. Biopsies of the vastus lateralis were collected from middle-aged Polynesian men and women with morbid obesity (44 kg/m(2) ± 10) and Type 2 diabetes before and following 16 wk of resistance (n = 9) or endurance training (n = 8). Longitudinal transcriptome, methylome, and microRNA (miRNA) responses were obtained via microarray, filtered by novel effect-size based false discovery rate probe selection preceding bioinformatic interrogation. Metabolic and microvascular transcriptome topology dominated the network landscape following endurance exercise. Lipid and glucose metabolism modules were connected to: microRNA (miR)-29a; promoter region hypomethylation of nuclear receptor factor (NRF1) and fatty acid transporter (SLC27A4), and hypermethylation of fatty acid synthase, and to exon hypomethylation of 6-phosphofructo-2-kinase and Ser/Thr protein kinase. Directional change in the endurance networks was validated by lower intramyocellular lipid, increased capillarity, GLUT4, hexokinase, and mitochondrial enzyme activity and proteome. Resistance training also lowered lipid and increased enzyme activity and caused GLUT4 promoter hypomethylation; however, training was inconsequential to GLUT4, capillarity, and metabolic transcriptome. miR-195 connected to negative regulation of vascular development. To conclude, integrated molecular network modelling revealed differential DNA methylation and miRNA expression changes occur in skeletal muscle in response to chronic exercise training that are most pronounced with endurance training and topographically associated with functional metabolic and microvascular plasticity relevant to diabetes rehabilitation. | | | 25138607
 |
Autologous minced muscle grafts: a tissue engineering therapy for the volumetric loss of skeletal muscle.
Corona, BT; Garg, K; Ward, CL; McDaniel, JS; Walters, TJ; Rathbone, CR
American journal of physiology. Cell physiology
305
C761-75
2013
Show Abstract
Volumetric muscle loss (VML) results in a large void deficient in the requisite materials for regeneration for which there is no definitive clinical standard of care. Autologous minced muscle grafts (MG), which contain the essential components for muscle regeneration, may embody an ideal tissue engineering therapy for VML. The purpose of this study was to determine if orthotopic transplantation of MG acutely after VML in the tibialis anterior muscle of male Lewis rats promotes functional tissue regeneration. Herein we report that over the first 16 wk postinjury, MG transplantation 1) promotes remarkable regeneration of innervated muscle fibers within the defect area (i.e., de novo muscle fiber regeneration); 2) reduced evidence of chronic injury in the remaining muscle mass compared with nonrepaired muscles following VML (i.e., transplantation attenuated chronically upregulated transforming growth factor-β1 gene expression and the presence of centrally located nuclei in 30% of fibers observed in nonrepaired muscles); and 3) significantly improves net torque production (i.e., ∼55% of the functional deficit in nonrepaired muscles was restored). Additionally, voluntary wheel running was shown to reduce the heightened accumulation of extracellular matrix deposition observed within the regenerated tissue of MG-repaired sedentary rats 8 wk postinjury (collagen 1% area: sedentary vs. runner, ∼41 vs. 30%), which may have been the result of an augmented inflammatory response [i.e., M1 (CCR7) and M2 (CD163) macrophage expression was significantly greater in runner than sedentary MG-repaired muscles 2 wk postinjury]. These findings support further exploration of autologous minced MGs for the treatment of VML. | | | 23885064
 |
Spatiotemporal control of vascular endothelial growth factor expression using a heat-shock-activated, rapamycin-dependent gene switch.
Martín-Saavedra, FM; Wilson, CG; Voellmy, R; Vilaboa, N; Franceschi, RT
Human gene therapy methods
24
160-70
2013
Show Abstract
A major challenge in regenerative medicine is to develop methods for delivering growth and differentiation factors in specific spatial and temporal patterns, thereby mimicking the natural processes of development and tissue repair. Heat shock (HS)-inducible gene expression systems can respond to spatial information provided by localized heating, but are by themselves incapable of sustained expression. Conversely, gene switches activated by small molecules provide tight temporal control and sustained expression, but lack mechanisms for spatial targeting. Here we combine the advantages of HS and ligand-activated systems by developing a novel rapamycin-regulated, HS-inducible gene switch that provides spatial and temporal control and sustained expression of transgenes such as firefly luciferase and vascular endothelial growth factor (VEGF). This gene circuit exhibits very low background in the uninduced state and can be repeatedly activated up to 1 month. Furthermore, dual regulation of VEGF induction in vivo is shown to stimulate localized vascularization, thereby providing a route for temporal and spatial control of angiogenesis. | | | 23527589
 |
Multiscale distribution and bioaccumulation analysis of clofazimine reveals a massive immune system-mediated xenobiotic sequestration response.
Baik, J; Stringer, KA; Mane, G; Rosania, GR
Antimicrobial agents and chemotherapy
57
1218-30
2013
Show Abstract
Chronic exposure to some well-absorbed but slowly eliminated xenobiotics can lead to their bioaccumulation in living organisms. Here, we studied the bioaccumulation and distribution of clofazimine, a riminophenazine antibiotic used to treat mycobacterial infection. Using mice as a model organism, we performed a multiscale, quantitative analysis to reveal the sites of clofazimine bioaccumulation during chronic, long-term exposure. Remarkably, between 3 and 8 weeks of dietary administration, clofazimine massively redistributed from adipose tissue to liver and spleen. During this time, clofazimine concentration in fat and serum significantly decreased, while the mass of clofazimine in spleen and liver increased by greater than 10-fold. These changes were paralleled by the accumulation of clofazimine in the resident macrophages of the lymphatic organs, with as much as 90% of the clofazimine mass in spleen sequestered in intracellular crystal-like drug inclusions (CLDIs). The amount of clofazimine associated with CLDIs of liver and spleen macrophages disproportionately increased and ultimately accounted for a major fraction of the total clofazimine in the host. After treatment was discontinued, clofazimine was retained in spleen while its concentrations decreased in blood and other organs. Immunologically, clofazimine bioaccumulation induced a local, monocyte-specific upregulation of various chemokines and receptors. However, interleukin-1 receptor antagonist was also upregulated, and the acute-phase response pathways and oxidant capacity decreased or remained unchanged, marking a concomitant activation of an anti-inflammatory response. These experiments indicate an inducible, immune system-dependent, xenobiotic sequestration response affecting the atypical pharmacokinetics of a small molecule chemotherapeutic agent. | | | 23263006
 |
Endothelial Progenitors Exist within the Kidney and Lung Mesenchyme.
Sims-Lucas, S; Schaefer, C; Bushnell, D; Ho, J; Logar, A; Prochownik, E; Gittes, G; Bates, CM
PloS one
8
e65993
2013
Show Abstract
The renal endothelium has been debated as arising from resident hemangioblast precursors that transdifferentiate from the nephrogenic mesenchyme (vasculogenesis) and/or from invading vessels (angiogenesis). While the Foxd1-positive renal cortical stroma has been shown to differentiate into cells that support the vasculature in the kidney (including vascular smooth muscle and pericytes) it has not been considered as a source of endothelial cell progenitors. In addition, it is unclear if Foxd1-positive mesenchymal cells in other organs such as the lung have the potential to form endothelium. This study examines the potential for Foxd1-positive cells of the kidney and lung to give rise to endothelial progenitors. We utilized immunofluorescence (IF) and fluorescence-activated cell sorting (FACS) to co-label Foxd1-expressing cells (including permanently lineage-tagged cells) with endothelial markers in embryonic and postnatal mice. We also cultured FACsorted Foxd1-positive cells, performed in vitro endothelial cell tubulogenesis assays and examined for endocytosis of acetylated low-density lipoprotein (Ac-LDL), a functional assay for endothelial cells. Immunofluorescence and FACS revealed that a subset of Foxd1-positive cells from kidney and lung co-expressed endothelial cell markers throughout embryogenesis. In vitro, cultured embryonic Foxd1-positive cells were able to differentiate into tubular networks that expressed endothelial cell markers and were able to endocytose Ac-LDL. IF and FACS in both the kidney and lung revealed that lineage-tagged Foxd1-positive cells gave rise to a significant portion of the endothelium in postnatal mice. In the kidney, the stromal-derived cells gave rise to a portion of the peritubular capillary endothelium, but not of the glomerular or large vessel endothelium. These findings reveal the heterogeneity of endothelial cell lineages; moreover, Foxd1-positive mesenchymal cells of the developing kidney and lung are a source of endothelial progenitors that are likely critical to patterning the vasculature. | | | 23823180
 |




























 Antibody[191011-ALL].jpg)