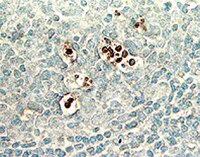
Effects of resveratrol on blood homocysteine level, on homocysteine induced oxidative stress, apoptosis and cognitive dysfunctions in rats.
Sema Tulay Koz,Ebru Onalan Etem,Gıyasettin Baydas,Huseyin Yuce,Halil Ibrahim Ozercan,Tuncay Kuloğlu,Suleyman Koz,Arzu Etem,Nevgul Demir
Brain research
1484
2012
Show Abstract
We aimed to examine the protective effects of resveratrol against homocysteine induced oxidative stress, apoptosis and cognitive impairment. Rats were randomly divided into three groups. Control group received standard rat food; homocysteine group (Hcy group) received daily methionine at a dose of 1g/kg-body weight dissolved in drinking water for thirty days; third group (Hcy+Res group) received same amount of methionine plus 20mg/kg/day resveratrol intraperitoneally for thirty days. Cognitive performances of the animals were tested by Morris water maze test. Then all animals were sacrificed to study lipid peroxidation (LPO), DNA fragmentation and p53 mRNA expression in the rat brain. The aortas of the sacrificed rats were processed for histopathological examination. Apoptosis in the aortas was assessed by TUNEL staining. Resveratrol significantly decreased serum levels of homocysteine, reversed Hcy induced LPO increase, decreased DNA fragmentation and p53 mRNA expression in the rat brains, and improved homocysteine induced impairment of long term spatial memory. Resveratrol could inhibit homocysteine induced apoptosis and histopathological deterioration in the rat aortic sections. In conclusion, resveratrol is effective in preventing homocysteine induced vascular and neural defects. In hyperhomocysteinemic rat model, our findings consequently warrant in future studies to reveal the true improvement mechanism of resveratrol. | 22995369
 |
SMARCAL1 deficiency predisposes to non-Hodgkin lymphoma and hypersensitivity to genotoxic agents in vivo.
Alireza Baradaran-Heravi,Anja Raams,Joanna Lubieniecka,Kyoung Sang Cho,Kristi A Dehaai,Mitra Basiratnia,Pierre-Olivier Mari,Yutong Xue,Michael Rauth,Ann Haskins Olney,Mary Shago,Kunho Choi,Rosanna A Weksberg,Malgorzata J M Nowaczyk,Weidong Wang,Nicolaas G J Jaspers,Cornelius F Boerkoel
American journal of medical genetics. Part A
158A
2012
Show Abstract
Schimke immuno-osseous dysplasia (SIOD) is a multisystemic disorder with prominent skeletal, renal, immunological, and ectodermal abnormalities. It is caused by mutations of SMARCAL1 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a-like 1), which encodes a DNA stress response protein. To determine the relationship of this function to the SIOD phenotype, we profiled the cancer prevalence in SIOD and assessed if defects of nucleotide excision repair (NER) and nonhomologous end joining (NHEJ), respectively, explained the ectodermal and immunological features of SIOD. Finally, we determined if Smarcal1(del/del) mice had hypersensitivity to irinotecan (CPT-11), etoposide, and hydroxyurea (HU) and whether exposure to these agents induced features of SIOD. Among 71 SIOD patients, three had non-Hodgkin lymphoma (NHL) and one had osteosarcoma. We did not find evidence of defective NER or NHEJ; however, Smarcal1-deficient mice were hypersensitive to several genotoxic agents. Also, CPT-11, etoposide, and HU caused decreased growth and loss of growth plate chondrocytes. These data, which identify an increased prevalence of NHL in SIOD and confirm hypersensitivity to DNA damaging agents in vivo, provide guidance for the management of SIOD patients. © 2012 Wiley Periodicals, Inc. | 22888040
 |
Effects of prolonged water washing of tissue samples fixed in formalin on histological staining.
Suzuki, Y; Imada, T; Yamaguchi, I; Yoshitake, H; Sanada, H; Kashiwagi, T; Takaba, K
Biotechnic & histochemistry : official publication of the Biological Stain Commission
87
241-8
2012
Show Abstract
The effects of prolonged water washing after fixation for 48 h in 10% (v/v) phosphate-buffered neutral formalin on the quality of representative histological staining methods were evaluated using samples of liver, kidney, spleen and thymus collected from three male Crl:CD(SD)(IGS) rats and one male beagle dog. Because door-to-door courier services in Japan prohibit handling formalin, our goal was to confirm that formalin fixed wet tissue samples could be stored in tap water rather than formalin during transportation of the samples without decreasing the quality of their staining or immunohistochemistry. Each tissue sample was allocated randomly to one of three groups: 12 min, 3 days and 7 days of washing in running tap water; samples then were routinely embedded in paraffin and sectioned. The sections were stained with hematoxylin and eosin, periodic acid-Schiff, azan, and the TdT-mediated dUTP-biotin nick end labeling (TUNEL) method. Immunohistochemical staining for Factor VIII, ED-1 and CD3 also was assessed. Prolonged water washing for up to 7 days did not affect the morphology or stainability by standard histological methods, or the intensity and frequency of positive reactions using the TUNEL method. Only immunohistochemical staining of Factor VIII was altered in both the rat and dog sections after 7 days of water washing. The intensity of positive reactions of Factor VIII immunohistochemistry after 7 days water washing was still strong enough to detect microscopically. Therefore, prolonged water washing for up to 7 days after formalin fixation does not have seriously detrimental effects on the quality and characteristics of paraffin sections stained by various methods, including immunohistochemistry. | 21958122
 |
Evaluation of apoptosis along with BCL-2 and Ki-67 expression in patients with intestinal metaplasia.
Gulbanu Erkan,Ipek Isik Gonul,Ugur Kandilci,Ayse Dursun
Pathology, research and practice
208
2012
Show Abstract
The primary aim is to compare individuals with intestinal metaplasia (IM), chronic active gastritis (CAG), and normal gastric mucosa (NGM) in terms of apoptosis, proliferation, and Bcl-2 expression. The secondary aim is to determine whether these parameters are different between patients with and without gastric cancer in first-degree relatives. We enrolled 106 patients whose histopathological results were consistent with IM (n: 42), CAG (n: 51), or NGM (n: 13). Antral biopsies were immunohistochemically stained for Bcl-2 and Ki-67 expression. Apoptosis was detected using TUNEL assay. While no significant difference was determined between three groups with regard to apoptosis and Bcl-2 expression (p>0.05), Ki-67 expression was significantly higher in the IM group when compared with the CAG and NGM groups (29.90±22.87 vs. 18.18±16.22 vs. 18.54±20, respectively; p=0.012). Helicobacter pylori was determined to increase apoptosis (49.3% vs. 25.7%, p<0.05), nevertheless, it had no significant effect on proliferation and Bcl-2 expression. Bcl-2 and Ki-67 expression and apoptosis were not different among patients with and without a history of gastric cancer in first degree relatives. Although intestinal metaplasia cases demonstrate an increase in proliferation, no elevation is observed in apoptosis. This can be an important factor in the progression to gastric cancer. | 22277792
 |
Does furan affect the thymus in growing male rats?
E Arzu Koçkaya,Aysun Kılıç,Elif Karacaoğlu,Güldeniz Selmanoğlu
Drug and chemical toxicology
35
2012
Show Abstract
Furan has been identified in foods such as heat-treated foods, including coffee, canned meat, hazelnuts, and infant foods and formulas. Children may be exposed to furan via either consumption of these foods or their derivatives. We evaluated the effects of furan on the thymus of weaning male rats in the present study. Five separate groups containing male rats were used: control, oil control, and three furan-treated groups. Furan was given orally to rats in the treatment groups at doses of 2, 4, and 8 mg/kg/day for 90 days. At the end of the experiment, thymus of the rats were examined morphologically, histopathologically, and immunohistochemically. We observed that absolute and relative weights of thymus were decreased significantly in rats treated with 4- and 8-mg/kg/day doses of furan. In histopathological examination, enlargement of interstitial connective tissue between the thymic lobules, lymphocyte depletion, and hemorrhage were observed. We detected an increase in apoptotic cell counts in thymus of the treatment groups. In addition, we found significant differences in the distribution of fibronectin and transforming growth factor-beta in the thymus of the treatment groups. In conclusion, we suggest that furan has affected the thymus in growing male rats. | 22289615
 |
Alteration of hepatic structure and oxidative stress induced by intravenous nanoceria.
Michael T Tseng,Xiaoqin Lu,Xiaoxian Duan,Sarita S Hardas,Rukhsana Sultana,Peng Wu,Jason M Unrine,Uschi Graham,D Allan Butterfield,Eric A Grulke,Robert A Yokel
Toxicology and applied pharmacology
260
2012
Show Abstract
Beyond the traditional use of ceria as an abrasive, the scope of nanoceria applications now extends into fuel cell manufacturing, diesel fuel additives, and for therapeutic intervention as a putative antioxidant. However, the biological effects of nanoceria exposure have yet to be fully defined, which gave us the impetus to examine its systemic biodistribution and biological responses. An extensively characterized nanoceria (5 nm) dispersion was vascularly infused into rats, which were terminated 1 h, 20 h or 30 days later. Light and electron microscopic tissue characterization was conducted and hepatic oxidative stress parameters determined. We observed acute ceria nanoparticle sequestration by Kupffer cells with subsequent bioretention in parenchymal cells as well. The internalized ceria nanoparticles appeared as spherical agglomerates of varying dimension without specific organelle penetration. In hepatocytes, the agglomerated nanoceria frequently localized to the plasma membrane facing bile canaliculi. Hepatic stellate cells also sequestered nanoceria. Within the sinusoids, sustained nanoceria bioretention was associated with granuloma formations comprised of Kupffer cells and intermingling CD3⁺ T cells. A statistically significant elevation of serum aspartate aminotransferase (AST) level was seen at 1 and 20 h, but subsided by 30 days after ceria administration. Further, elevated apoptosis was observed on day 30. These findings, together with increased hepatic protein carbonyl levels on day 30, indicate ceria-induced hepatic injury and oxidative stress, respectively. Such observations suggest a single vascular infusion of nanoceria can lead to persistent hepatic retention of particles with possible implications for occupational and therapeutic exposures. | 22373796
 |
Early regression of the dental lamina underlies the development of diphyodont dentitions.
M Buchtová,J Stembírek,K Glocová,E Matalová,A S Tucker
Journal of dental research
91
2012
Show Abstract
Functional tooth germs in mammals, reptiles, and chondrichthyans are initiated from a dental lamina. The longevity of the lamina plays a role in governing the number of tooth generations. Monophyodont species have no replacement dental lamina, while polyphyodont species have a permanent continuous lamina. In diphyodont species, the dental lamina fragments and regresses after initiation of the second tooth generation. Regression of the lamina seems to be an important mechanism in preventing the further development of replacement teeth. Defects in the complete removal of the lamina lead to cyst formation and has been linked to ameloblastomas. Here, we show the previously unknown mechanisms behind the disappearance of the dental lamina, involving a combination of cell migration, cell-fate transformation, and apoptosis. Lamina regression starts with the loss of the basement membrane, allowing the epithelial cells to break away from the lamina and migrate into the surrounding mesenchyme. Cells deactivate epithelial markers (E-cadherin, cytokeratin), up-regulate Slug and MMP2, and activate mesenchymal markers (vimentin), while residual lamina cells are removed by apoptosis. The uncovering of the processes behind lamina degradation allows us to clarify the evolution of diphyodonty, and provides a mechanism for future manipulation of the number of tooth generations. | 22442052
 |
Compartmentalization and regulation of iron metabolism proteins protect male germ cells from iron overload.
Yael Leichtmann-Bardoogo,Lyora A Cohen,Avital Weiss,Britta Marohn,Stephanie Schubert,Andreas Meinhardt,Esther G Meyron-Holtz
American journal of physiology. Endocrinology and metabolism
302
2012
Show Abstract
The universal importance of iron, its high toxicity, and complex chemistry present a challenge to biological systems in general and to protected compartments in particular. The high mitotic rate and avid mitochondriogenesis of developing male germ cells imply high iron requirements. Yet access to germ cells is tightly regulated by the blood-testis barrier that protects the meiotic and postmeiotic germ cells. To elucidate how iron is supplied to developing male germ cells, we analyzed iron deposition and iron transport proteins in testes of mice with iron overload and with genetic ablation of the iron regulators Hfe and iron regulatory protein 2. Iron accumulated mainly around seminiferous tubules, and only small amounts localized within the seminiferous tubules. The localization and regulation of proteins involved in iron import, storage, and export such as transferrin, transferrin receptor, the divalent metal transporter-1, cytosolic ferritin, and ferroportin strongly support a model of a largely autonomous iron cycle within seminiferous tubules. We show evidence that ferritin secretion from Sertoli cells may play an important role in iron acquisition of primary spermatocytes. During spermatogenic development iron is carried along from primary spermatocytes to spermatids, and from spermatids iron is recycled to the apical compartment of Sertoli cells, which traffic it back to a new generation of spermatocytes. Losses are replenished by the peripheral circulation. Such an internal iron cycle essentially detaches the iron homeostasis within the seminiferous tubule from the periphery and protects developing germ cells from iron fluctuations. This model explains how compartmentalization can optimize cellular and systemic nutrient homeostasis. | 22496346
 |
Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine.
Kamal Datta,Shubhankar Suman,Bhaskar V S Kallakury,Albert J Fornace
PloS one
7
2012
Show Abstract
Ionizing radiation-induced oxidative stress is attributed to generation of reactive oxygen species (ROS) due to radiolysis of water molecules and is short lived. Persistent oxidative stress has also been observed after radiation exposure and is implicated in the late effects of radiation. The goal of this study was to determine if long-term oxidative stress in freshly isolated mouse intestinal epithelial cells (IEC) is dependent on radiation quality at a dose relevant to fractionated radiotherapy. Mice (C57BL/6J; 6 to 8 weeks; female) were irradiated with 2 Gy of γ-rays, a low-linear energy transfer (LET) radiation, and intestinal tissues and IEC were collected 1 year after radiation exposure. Intracellular ROS, mitochondrial function, and antioxidant activity in IEC were studied by flow cytometry and biochemical assays. Oxidative DNA damage, cell death, and mitogenic activity in IEC were assessed by immunohistochemistry. Effects of γ radiation were compared to (56)Fe radiation (iso-toxic dose: 1.6 Gy; energy: 1000 MeV/nucleon; LET: 148 keV/ | 22936983
 |
Biomarkers of phenethyl isothiocyanate-mediated mammary cancer chemoprevention in a clinically relevant mouse model.
Shivendra V Singh,Su-Hyeong Kim,Anuradha Sehrawat,Julie A Arlotti,Eun-Ryeong Hahm,Kozue Sakao,Jan H Beumer,Rachel C Jankowitz,Kumar Chandra-Kuntal,Joomin Lee,Anna A Powolny,Rajiv Dhir
Journal of the National Cancer Institute
104
2012
Show Abstract
Phenethyl isothiocyanate (PEITC) is a natural plant compound with chemopreventative potential against some cancers and the ability to induce apoptosis in breast cancer cells. | 22859850
 |