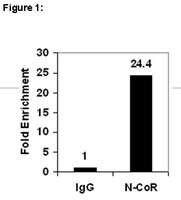
SUMOylation of the corepressor N-CoR modulates its capacity to repress transcription.
Tiefenbach, Jens, et al.
Mol. Biol. Cell, 17: 1643-51 (2006)
2006
Show Abstract
In the absence of ligands the corepressor N-CoR mediates transcriptional repression by some nuclear hormone receptors. Several protein-protein interactions of N-CoR are known, of which mainly complex formation with histone deacetylases (HDACs) leads to the repression of target genes. On the other hand, the role of posttranslational modifications in corepressor function is not well established. Here, we show that N-CoR is modified by Sumo-1. We found SUMO-E2-conjugating enzyme Ubc9 and SUMO-E3 ligase Pias1 as novel N-CoR interaction partners. The SANT1 domain of N-CoR was found to mediate this interaction. We show that K152, K1117, and K1330 of N-CoR can be conjugated to SUMO and that mutation of all sites is necessary to fully block SUMOylation in vitro. Because these lysine residues are located within repression domains I and III, respectively, we investigated a possible correlation between the functions of the repression domains and SUMOylation. Coexpression of Ubc9 protein resulted in enhanced N-CoR-dependent transcriptional repression. Studies using SUMOylation-deficient N-CoR RDI mutants suggest that SUMO modification contributes to repression by N-CoR. Mutation of K152 to R in RD1, for example, not only significantly reduced repression of a reporter gene, but also abolished the effect of Ubc9 on transcriptional repression. | 16421255
 |
A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and the corepressor KAP-1.
Underhill, C, et al.
J. Biol. Chem., 275: 40463-70 (2000)
2000
Show Abstract
Transcriptional silencing by many transcription factors is mediated by the nuclear receptor corepressor (N-CoR). The mechanism by which N-CoR represses basal transcription involves the direct or indirect recruitment of histone deacetylases (HDACs). We have isolated two multiprotein N-CoR complexes, designated N-CoR-1 and N-CoR-2, which possess histone deacetylase activity that is mediated by distinct HDACs. Based on Western blotting using antibodies against known subunits, the only HDAC found in the N-CoR-1 complex was HDAC3. In contrast, N-CoR-2 contained predominantly HDAC1 and HDAC2 as well as several other subunits that are found in the Sin3A.HDAC complex. Using mass spectrometry and Western blotting, we have identified several novel components of the N-CoR-1 complex including the SWI/SNF-related proteins BRG1, BAF 170, BAF 155, BAF 47/INI1, and the corepressor KAP-1 that is involved in silencing heterochromatin. Indirect immunofluorescence has revealed that both KAP-1 and N-CoR colocalize throughout the nucleus. These results suggest that N-CoR is found in distinct multiprotein complexes, which are involved in multiple pathways of transcriptional repression. | 11013263
 |
The corepressor N-CoR and its variants RIP13a and RIP13Delta1 directly interact with the basal transcription factors TFIIB, TAFII32 and TAFII70.
Muscat, G E, et al.
Nucleic Acids Res., 26: 2899-907 (1998)
1998
Show Abstract
Repression of transcription by the classical nuclear receptors (e.g. TR, RAR), the orphan nuclear receptors (e.g. Rev-erbAalpha/beta), Mxi-1 and Mad bHLH-zip proteins and the oncoproteins PLZF and LAZ3/BCL6 is mediated by the corepressors N-CoR and SMRT. The interaction of the corepressors with the components involved in chromatin remodelling, such as the recruiting proteins Sin3A/B and the histone deacteylases HDAc-1 and RPD3, has been analysed in detail. The N-CoR/Sin3/HDAc complexes have a key role in the regulation of cellular proliferation and differentiation. However, the interaction of these corepressors with the basal transcriptional machinery has remained obscure. In this study we demonstrated that the N-terminalrepression domains and the receptor interactiondomains (RID) of N-CoR and its splice variants, RIP13a and RIP13Delta1, directly interact with TAFII32 in vivo and in vitro . We show that interaction domain II within the N-CoR and RIP13a RID is required for the interaction with TAFII32. We also observed that N-CoR directly interacts with each of the basal factors, TFIIB and TAFII70, and can simultaneously interact with all three basal factors in a non-competitive manner. Furthermore, we provide evidence that suggests the RVR/Rev-erbbeta-corepressor complex also interacts with the general transcriptional machinery, and that the physicalassociation of TFIIB with N-CoR also occurs in the presence of Sin3B and HDAc-1. Interestingly, we observed that N-CoR expression ablated the functional interaction between TFIIB and TAFII32 that is critical to the initiation of transcription. In conclusion, this study demonstrates that the N-terminal repressor region and the C-terminal RIDs are part of the corepressor contact interface that mediates the interaction with the general transcription factors, and demonstrates that TAFs can also directly interact with corepressors to mediate signals from repressors to the basal machinery. We also suggest that N-CoR interacts with the central components of the transcriptional initiation process (TFIIB, TAFs) and locks them into a non-functional complex or conformation that is not conducive to transcription. | 9611234
 |