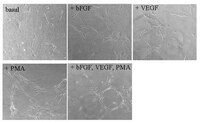
Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis.
Robert A Boomsma,David L Geenen
PloS one
7
2012
Show Abstract
We have previously shown that mesenchymal stem cells (MSC) improve function upon integration in ischemic myocardium. We examined whether specific cytokines and growth factors produced by MSCs are able to affect angiogenesis, cellular migration and apoptosis. Conditioned media (CM) was prepared by culturing MSC for 48 hours. CM displayed significantly elevated levels of VEGF, Monocyte Chemoattractant Protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1β and monokine induced by IFN-γ (MIG) compared to control media. MSC contained RNA for these factors as detected by RT-PCR. CM was able to induce angiogenesis in canine vascular endothelial cells. MCP-1 and MIP-1α increased cell migration of MSC while VEGF reduced it. H9c2 cells treated with CM under hypoxic conditions for 24 hours displayed a 16% reduction in caspase-3 activity compared to controls. PI 3-kinase γ inhibitor had no effect on controls but reversed the effect of CM on caspase-3 activity. MCP-1 alone mimicked the protective effect of CM while the PI 3-Kγ inhibitor did not reverse the effect of MCP-1. CM reduced phospho-BAD (Ser112) and phospho-Akt (Ser473) while increasing phospho-Akt (Thr308). MCP-1 reduced the level of phospho-Akt (Ser473) while having no effect on the other two; the PI 3-Kγ inhibitor did not alter the MCP-1 effect. ERK 1/2 phosphorylation was reduced in CM treated H9c2 cells, and inhibition of ERK 1/2 reduced the phosphorylation of Akt (Ser473), Akt (Thr308) and Bad (Ser112). In conclusion, MSC synthesize and secrete multiple paracrine factors that are able to affect MSC migration, promote angiogenesis and reduce apoptosis. While both MCP-1 and PI3-kinase are involved in the protective effect, they are independent of each other. It is likely that multiple pro-survival factors in addition to MCP-1 are secreted by MSC which act on divergent intracellular signaling pathways. | 22558198
 |
High-throughput production of gene replacement mutants in Neurospora crassa.
Gyungsoon Park,Hildur V Colot,Patrick D Collopy,Svetlana Krystofova,Christopher Crew,Carol Ringelberg,Liubov Litvinkova,Lorena Altamirano,Liande Li,Susan Curilla,Wei Wang,Norma Gorrochotegui-Escalante,Jay C Dunlap,Katherine A Borkovich
Methods in molecular biology (Clifton, N.J.)
722
2011
Show Abstract
The model filamentous fungus Neurospora crassa has been the focus of functional genomics studies for the past several years. A high-throughput gene knockout procedure has been developed and used to generate mutants for more than two-thirds of the ∼10,000 annotated N. crassa genes. Yeast recombinational cloning was incorporated as an efficient procedure to produce all knockout cassettes. N. crassa strains with the Δmus-51 or Δmus-52 deletion mutations were used as transformation recipients in order to reduce the incidence of ectopic integration and increase homologous recombination of knockout cassettes into the genome. A 96-well format was used for many steps of the procedure, including fungal transformation, isolation of homokaryons, and verification of mutants. In addition, development of software programs for primer design and restriction enzyme selection facilitated the high-throughput aspects of the overall protocol. | 21590421
 |
The transcriptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a {beta}-secretase-dependent pathway.
Nikolai D Belyaev,Katherine A B Kellett,Caroline Beckett,Natalia Z Makova,Timothy J Revett,Natalia N Nalivaeva,Nigel M Hooper,Anthony J Turner
The Journal of biological chemistry
285
2010
Show Abstract
Amyloidogenic processing of the amyloid precursor protein (APP) by β- and γ-secretases generates several biologically active products, including amyloid-β (Aβ) and the APP intracellular domain (AICD). AICD regulates transcription of several neuronal genes, especially the Aβ-degrading enzyme, neprilysin (NEP). APP exists in several alternatively spliced isoforms, APP(695), APP(751), and APP(770). We have examined whether each isoform can contribute to AICD generation and hence up-regulation of NEP expression. Using SH-SY5Y neuronal cells stably expressing each of the APP isoforms, we observed that only APP(695) up-regulated nuclear AICD levels (9-fold) and NEP expression (6-fold). Increased NEP expression was abolished by a β- or γ-secretase inhibitor but not an α-secretase inhibitor. This correlated with a marked increase in both Aβ(1-40) and Aβ(1-42) in APP(695) cells as compared with APP(751) or APP(770) cells. Similar phenomena were observed in Neuro2a but not HEK293 cells. SH-SY5Y cells expressing the Swedish mutant of APP(695) also showed an increase in Aβ levels and NEP expression as compared with wild-type APP(695) cells. Chromatin immunoprecipitation revealed that AICD was associated with the NEP promoter in APP(695), Neuro2a, and APP(Swe) cells but not APP(751) nor APP(770) cells where AICD was replaced by histone deacetylase 1 (HDAC1). AICD occupancy of the NEP promoter was replaced by HDAC1 after treatment of the APP(695) cells with a β- but not an α-secretase inhibitor. The increased AICD and NEP levels were significantly reduced in cholesterol-depleted APP(695) cells. In conclusion, Aβ and functional AICD appear to be preferentially synthesized through β-secretase action on APP(695). Full Text Article | 20961856
 |
Coupling aerobic biodegradation of methanol vapors with heterologous protein expression of endochitinase Ech42 from Trichoderma atroviride in Pichia pastoris.
Sonia Arriaga,Julia A Acosta-Munguía,Ana S Pérez-Martínez,Antonio De León-Rodríguez,Ana P Barba de la Rosa
Bioresource technology
101
2010
Show Abstract
Methanol is included among the most hazardous air pollutants, and an effort of vapors biofiltration by using microbial consortiums has been reported. The aim of this work was to couple the methanol vapors biodegradation with the production of recombinant endochitinase (ech42) from Trichoderma atroviride in Pichia pastoris transformed with the pPIC-ech42 plasmid. After carrying out batch experiments at 0.5% (w/v) of methanol concentration, the recombinant P. pastoris Mut(+) strain was selected because it showed methanol biodegradation rates similar to those of wild type GS115 strain (39 g/m(3)h), but 15% higher than the transformed Mut(S) strain. In addition, the recombinant Ech42 protein production was higher in Mut(+) than Mut(S). After various methanol vapor concentrations were evaluated, the maximum recombinant protein recovery was 317 mg/l and the volumetric methanol consumption rate was 88.7 g/m(3)h at 0.5% (w/v) of methanol concentration. This research underlines the promising application of linking methanol vapors biodegradation with the production of recombinant protein with high biotechnological interests. | 20709543
 |
Direct interactions of intraflagellar transport complex B proteins IFT88, IFT52, and IFT46.
Ben F Lucker,Mark S Miller,Slawomir A Dziedzic,Philip T Blackmarr,Douglas G Cole
The Journal of biological chemistry
285
2010
Show Abstract
Intraflagellar transport (IFT) particles of Chlamydomonas reinhardtii contain two distinct protein complexes, A and B, composed of at least 6 and 15 protein subunits, respectively. As isolated from C. reinhardtii flagella, IFT complex B can be further reduced to a approximately 500-kDa core that contains IFT88, 2x IFT81, 2x IFT74/72, IFT52, IFT46, IFT27, IFT25, and IFT22. In this study, yeast-based two-hybrid analysis was combined with bacterial coexpression to show that three of the core B subunits, IFT88, IFT52, and IFT46, interact directly with each other and, together, are capable of forming a ternary complex. Chemical cross-linking results support the IFT52-IFT88 interaction and provide additional evidence of an association between IFT27 and IFT81. With previous studies showing that IFT81 and IFT74/72 interact to form a (IFT81)(2)(IFT74/72)(2) heterotetramer and that IFT27 and IFT25 form a heterodimer, the architecture of complex B is revealing itself. Last, electroporation of recombinant IFT46 was used to rescue flagellar assembly of a newly identified ift46 mutant and to monitor in vivo localization and movement of the IFT particles. Full Text Article | 20435895
 |
The anti-viral protein of trichosanthin penetrates into human immunodeficiency virus type 1.
Wenlong Zhao,Du Feng,Shan Sun,Ting Han,Senfang Sui
Acta biochimica et biophysica Sinica
42
2010
Show Abstract
Trichosanthin (TCS) is a type I ribosome-inactivating protein with potent inhibitory activity against human immunodeficiency virus type 1, and has been clinically applied in acquired immunodeficiency syndrome (AIDS) therapy. Previous studies revealed that TCS recognized human immunodeficiency virus type 1 (HIV-1) particles. Here, we investigated the physical relationship between TCS and HIV-1 particles, and demonstrated that TCS penetrates into viral particles, where it is protected from various protease digestion. The penetration of TCS exerts no obvious effect on viral integrity. FYY140-142, D176, and K177 were identified as key amino acid residues for the membranetranslocation process. Moreover, TCS penetrated into HIV-1 virions showed potent anti-viral activity. Overall, the observations suggest that the penetration of TCS into HIV-1 particles may be important for eliminating the virus. | 20119629
 |
Subcellular localization and expression of bamboo mosaic virus satellite RNA-encoded protein.
Palani, PV; Chiu, M; Chen, W; Wang, CC; Lin, CC; Hsu, CC; Cheng, CP; Chen, CM; Hsu, YH; Lin, NS
The Journal of general virology
90
507-18
2009
Show Abstract
The satellite RNA of bamboo mosaic virus (satBaMV) has a single open reading frame encoding a non-structural protein, P20, which facilitates long-distance movement of satBaMV in BaMV and satBaMV co-infected plants. Immunohistochemistry and immunoelectron microscopy revealed that the P20 protein accumulated in the cytoplasm and nuclei in co-infected cells. P20 and the helper virus coat protein (CP) were highly similar in their subcellular localization, except that aggregates of BaMV virions were not labelled with anti-P20 serum. The BaMV CP protein was fairly abundant in mesophyll cells, whilst P20 was more frequently detected in mesophyll cells and vascular tissues. The expression kinetics of the P20 protein was similar to but slightly earlier than that of CP in co-infected Bambusa oldhamii protoplasts and Nicotiana benthamiana leaves. However, satBaMV-encoded protein levels declined rapidly in the late phase of co-infection. During co-infection, in addition to the intact P20, a low-molecular-mass polypeptide of 16 kDa was identified as a P20 C-terminally truncated product; the possible method of generation of the truncated protein is discussed. Full Text Article | 19141462
 |
A novel sorting strategy of trichosanthin for hijacking human immunodeficiency virus type 1.
Wen-Long Zhao,Fan Zhang,Du Feng,Ju Wu,Shan Chen,Sen-Fang Sui
Biochemical and biophysical research communications
384
2009
Show Abstract
Trichosanthin (TCS) is a type I ribosome-inactivating protein that plays dual role of plant toxin and anti-viral peptide. The sorting mechanism of such an exogenous protein is in long pursuit. Here, we examined TCS trafficking in cells expressing the HIV-1 scaffold protein Gag, and we found that TCS preferentially targets the Gag budding sites at plasma membrane or late endosomes depending on cell types. Lipid raft membrane but not the Gag protein mediates the association of TCS with viral components. After Gag budding, TCS is then released in association with the virus-like particles to generate TCS-enriched virions. The resulting TCS-enriched HIV-1 exhibits severely impaired infectivity. Overall, the observations indicate the existence of a unique and elaborate sorting strategy for hijacking HIV-1. | 19409877
 |
Engineering of a femtomolar affinity binding protein to human serum albumin.
Andreas Jonsson,Jakob Dogan,Nina Herne,Lars Abrahmsén,Per-Ake Nygren
Protein engineering, design & selection : PEDS
21
2008
Show Abstract
We describe the development of a novel serum albumin binding protein showing an extremely high affinity (K(D)) for HSA in the femtomolar range. Using a naturally occurring 46-residue three-helix bundle albumin binding domain (ABD) of nanomolar affinity for HSA as template, 15 residues were targeted for a combinatorial protein engineering strategy to identify variants showing improved HSA affinities. Sequencing of 55 unique phage display-selected clones showed a strong bias for wild-type residues at nine positions, whereas various changes were observed at other positions, including charge shifts. Additionally, a few non-designed substitutions appeared. On the basis of the sequences of 12 variants showing high overall binding affinities and slow dissociation rate kinetics, a set of seven 'second generation' variants were constructed. One variant denoted ABD035 displaying wild-type-like secondary structure content and excellent thermal denaturation/renaturation properties showed an apparent affinity for HSA in the range of 50-500 fM, corresponding to several orders of magnitude improvement compared with the wild-type domain. The ABD035 variant also showed an improved affinity toward serum albumin from a number of other species, and a capture experiment involving human serum indicated that the selectivity for serum albumin had not been compromised from the affinity engineering. | 18499681
 |
Recombinant norovirus-specific scFv inhibit virus-like particle binding to cellular ligands.
Khalil Ettayebi,Michele E Hardy
Virology journal
5
2008
Show Abstract
Noroviruses cause epidemic outbreaks of gastrointestinal illness in all age-groups. The rapid onset and ease of person-to-person transmission suggest that inhibitors of the initial steps of virus binding to susceptible cells have value in limiting spread and outbreak persistence. We previously generated a monoclonal antibody (mAb) 54.6 that blocks binding of recombinant norovirus-like particles (VLP) to Caco-2 intestinal cells and inhibits VLP-mediated hemagglutination. In this study, we engineered the antigen binding domains of mAb 54.6 into a single chain variable fragment (scFv) and tested whether these scFv could function as cell binding inhibitors, similar to the parent mAb. Full Text Article | 18237416
 |