345701 Sigma-AldrichGambogic Acid, Garcinia hanburyi - CAS 2752-65-0 - Calbiochem
A cell-permeable caspase activator and an apoptosis inducer that was originally isolated from gamboge resin for its antimicrobial properties.
More>> A cell-permeable caspase activator and an apoptosis inducer that was originally isolated from gamboge resin for its antimicrobial properties. Less<<Synonyms: Guttic Acid, β-Guttiferin
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 2752-65-0 | C₃₈H₄₄O₈ |
Products
| Catalog Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 345701-25MG | Alu drum | 25 mg |
| References | |
|---|---|
| References | Prasad, S., et al. 2011. Cancer Prev. Res. 4, 1084. Guo, Q.L., et al. 2004. Acta Pharmacol. Sin. 25, 769. Asano, J., et al. 1996. Phytochemistry 41, 815. |
| Product Information | |
|---|---|
| CAS number | 2752-65-0 |
| ATP Competitive | N |
| Form | Amorphous orange solid |
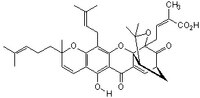
| Hill Formula | C₃₈H₄₄O₈ |
| Chemical formula | C₃₈H₄₄O₈ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Caspase activator and an apoptosis inducer |
| Primary Target IC<sub>50</sub> | MIC = 12.5 µg/ml in inhibiting the growth of HeLa and HEL cells |
| Purity | ≥95% by TLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | PB9268200 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 345701-25MG | 04055977193787 |
Documentation
Gambogic Acid, Garcinia hanburyi - CAS 2752-65-0 - Calbiochem SDS
| Title |
|---|
Gambogic Acid, Garcinia hanburyi - CAS 2752-65-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 345701 |
References
| Reference overview |
|---|
| Prasad, S., et al. 2011. Cancer Prev. Res. 4, 1084. Guo, Q.L., et al. 2004. Acta Pharmacol. Sin. 25, 769. Asano, J., et al. 1996. Phytochemistry 41, 815. |