345700 Sigma-AldrichGanciclovir - CAS 82410-32-0 - Calbiochem
Ganciclovir, CAS 82410-32-0, is a nucleoside analog related to Acyclovir. Acts as a prodrug that is activated by phosphorylation. Inhibits the replication of cytomegalovirus (CMV; IC50 = 10 nM).
More>> Ganciclovir, CAS 82410-32-0, is a nucleoside analog related to Acyclovir. Acts as a prodrug that is activated by phosphorylation. Inhibits the replication of cytomegalovirus (CMV; IC50 = 10 nM). Less<<Synonyms: 9-[(1,3-Dihydroxy-2-propoxy)methyl]guanine
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 82410-32-0 | C₉H₁₃N₅O₄ |
Products
| Catalog Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 345700-50MG | Glass bottle | 50 mg |
| Description | |
|---|---|
| Overview | A nucleoside analog structurally related to Acyclovir (Cat. No. 114798). Acts as a prodrug that is activated by phosphorylation. Has been used in the study of “suicide” gene therapy in cancer research. Upon expression of a viral suicide gene encoding herpes simplex virus, thymidine kinase (TK), the non-toxic prodrug ganciclovir, is converted to an active phosphorylated analog that can be incorporated into the DNA of replicating eukaryotic cells, causing death of the malignant dividing cell. Causes an irreversible cell cycle arrest at the G2/M checkpoint. Has also been used to study the loss of telomeres and to evaluate sensitivity of viruses to antiviral agents. |
| Catalogue Number | 345700 |
| Brand Family | Calbiochem® |
| Synonyms | 9-[(1,3-Dihydroxy-2-propoxy)methyl]guanine |
| Product Information | |
|---|---|
| CAS number | 82410-32-0 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₉H₁₃N₅O₄ |
| Chemical formula | C₉H₁₃N₅O₄ |
| Reversible | N |
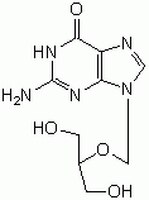
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | Acts as a prodrug that is activated by phosphorylation |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | MF8407000 |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 345700-50MG | 04055977194500 |
Documentation
Ganciclovir - CAS 82410-32-0 - Calbiochem SDS
| Title |
|---|
Ganciclovir - CAS 82410-32-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 345700 |
References
| Reference overview |
|---|
| Aghi, M., et al. 2000. J. Gene Med. 2, 148. Qiao, J., et al. 2000. Hum. Gene Ther. 11, 1569. Cannon, J.S., et al. 1999. J. Virol. 73, 4786. Rubsam, L.Z., et al. 1999. Cancer Res. 59, 669. Sprung, C.N., et al. 1999. Proc. Natl. Acad. Sci. USA 96, 6781. Yamasaki, H., et al. 1999. C.R. Acad. Sci. III 322, 151. Halloran, P.J., and Fenton, R.G. 1998. Cancer Res. 58, 3855. |