506306 Sigma-AldrichIKKε/TBK1 Inhibitor II, MRT67307 - CAS 1190378-57-4 - Calbiochem
A cell-permeable, potent, ATP-competitive, and reversible dual kinase inhibitor of TBK1/IKKε (IC₅₀ = 19 and 160 nM, respectively).
More>> A cell-permeable, potent, ATP-competitive, and reversible dual kinase inhibitor of TBK1/IKKε (IC₅₀ = 19 and 160 nM, respectively). Less<<Synonyms: TANK Binding Kinase 1/IKKinducible Inhibitor II, N-(3-(5-Cyclopropyl-2-(3-(morpholinomethyl)phenylamino)pyrimidin-4-ylamino)propyl)cyclobutanecarboxamide
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 1190378-57-4 | C₂₆H₃₆N₆O₂ |
Products
| Catalog Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.06306.0001 | Glass bottle | 5 mg |
| Description | |
|---|---|
| Overview | A cell-permeable BX795 analog (Cat. No. 204001) that acts as a potent, ATP-competitive, and reversible dual kinase inhibitor of TBK1/IKKε (IC50 = 19 and 160 nM, respectively) with excellent selectivity over IKKα and IKKβ (IC50 > 10 µM). Interacts with the TBK1 kinase dimer interface and stabilizes the inactive DFG-out conformation. Also blocks the activity of MARK (microtubule- associated protein (MAP)-microtubule affinity regulating kinase) 1, 2, 3, and 4 (IC50 = 27, 52, 36, and 41 nM, respectively), SIK2 (IC50 = 67 nM) and Aurora B, JAK2, and MLK1,3 (> than 90% inhibition at 1 µM) in a 108-kinase panel. Increases TNF-α-stimulated NF-κB-dependent gene transcription in wild-type macrophages and enhances CREB-dependent gene transcription by promoting dephosphorylation of CREB-regulated transcription coactivator (CRTC3). Shown to aid TLR-stimulated production of anti-Inflammatory cytokines in macrophages while suppressing the secretion of pro-inflammatory cytokines. In response to pro-inflammatory stimuli, pre-treatment of BX795 robustly suppresses the activation of JNK and p38α, whereas MRT67307 does not exhibit such off-target effects. Please note that the molecular weight for this compound is batch-specific due to variable water content. Please refer to the vial label or the certificate of analysis for the batch-specific molecular weight. The molecular weight provided represents the baseline molecular weight without water and salt. |
| Catalogue Number | 506306 |
| Brand Family | Calbiochem® |
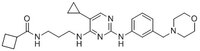
| Synonyms | TANK Binding Kinase 1/IKKinducible Inhibitor II, N-(3-(5-Cyclopropyl-2-(3-(morpholinomethyl)phenylamino)pyrimidin-4-ylamino)propyl)cyclobutanecarboxamide |
| Product Information | |
|---|---|
| CAS number | 1190378-57-4 |
| Form | Amber solid |
| Hill Formula | C₂₆H₃₆N₆O₂ |
| Chemical formula | C₂₆H₃₆N₆O₂ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | TBK1 kinase dimer interface |
| Secondary target | IKKe, MARK, SIK |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 5.06306.0001 | 04055977243253 |
Documentation
IKKε/TBK1 Inhibitor II, MRT67307 - CAS 1190378-57-4 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Bruni, D., et al. 2013. J. Immunol. 190, 2844. Tu, D., et al. Cell Reports 3, 747. Larabi, A., et al. Cell Reports 3, 734. Clark, K., et al. 2012. Proc. Natl. Acad. Sci. USA 109, 16986. Clark, K., et al. 2011. Proc. Natl. Acad. Sci. USA 108, 17093. Clark, K., et al. 2011. Biochem. J. 434, 93. |
| Data Sheet | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|