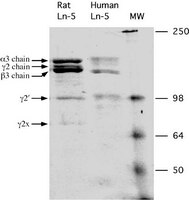
The epithelium-tooth interface--a basal lamina rich in laminin-5 and lacking other known laminin isoforms.
Hormia, M, et al.
J. Dent. Res., 77: 1479-85 (1998)
1998
Show Abstract
The attachment of the marginal gingiva to the tooth surface is mediated by a thin nonkeratinized epithelium termed the junctional epithelium (JE). Ultrastructural studies have revealed that the attachment of the JE to the tooth surface occurs through hemidesmosomes (HD) and a basal lamina-like extracellular matrix termed the internal basal lamina (IBL). We have previously shown that neither type IV collagen nor prototypic laminin, two common components of basement membranes (BM), is present in the IBL between the epithelium and the tooth. In the present study, we show that laminin-5 is a major component of the IBL in both rodent and human tissues. By using in situ hybridization, we also show that the cells of the JE express the LAMC2 gene of laminin-5. In other parts of gingival epithelium, LAMC2 gene expression is less prominent. Our results indicate that the epithelium-tooth interface is a unique structure wherein epithelial cells are induced to secrete a basal lamina containing laminin-5 and no other presently known laminin isoform. | 9663432
 |
Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5.
Giannelli, G, et al.
Science, 277: 225-8 (1997)
1997
Show Abstract
Structural changes in the extracellular matrix are necessary for cell migration during tissue remodeling and tumor invasion. Specific cleavage of laminin-5 (Ln-5) by matrix metalloprotease-2 (MMP2) was shown to induce migration of breast epithelial cells. MMP2 cleaved the Ln-5 gamma2 subunit at residue 587, exposing a putative cryptic promigratory site on Ln-5 that triggers cell motility. This altered form of Ln-5 is found in tumors and in tissues undergoing remodeling, but not in quiescent tissues. Cleavage of Ln-5 by MMP2 and the resulting activation of the Ln-5 cryptic site may provide new targets for modulation of tumor cell invasion and tissue remodeling. | 9211848
 |