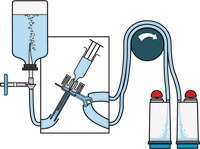
TZHASY210 MilliporeSteritest™ NEO devices for liquids in syringes mixed cellulose ester membrane 0,45µm 10/pk
Recommended Products
Overview
| Replacement Information |
|---|
| References |
|---|
| Product Information | |
|---|---|
| HS Code | 8421 29 90 |
| Device Configuration | 2 canisters |
| Filter Color | White |
| Color Code | Blue |
| Maximum Inlet Pressure, bar (psi) | 3.1 bar (45 psi) @ 25 °C |
| Maximum Operating Temperature | 45 °C |
| Type of Test |
|
| Quality Level | MQ400 |
| Applications | |
|---|---|
| Application | The Steritest™ NEO Device for pre-filled syringes is used for sterility testing of pre-filled syringes. |
| Key Applications |
|
| Biological Information | |
|---|---|
| Media | MF-Millipore |
| Sterility | Sterile |
| Sterilization | Gamma irradiation |
| Physicochemical Information | |
|---|---|
| Pore Size | 0.45 µm |
| Dimensions | |
|---|---|
| Filter Surface | Plain |
| Volume | 120 mL |
| Filter Diameter (⌀) | 47 mm |
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements | |
|---|---|
| Regulatory Conformance | Sterility Testing USP 71, EP2.6.1 , JP 4.06 |
| Storage and Shipping Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 10 |
| Material Package | 10 blisters per box, single packed |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| TZHASY210 | 04053252297793 |
Documentation
Steritest™ NEO devices for liquids in syringes mixed cellulose ester membrane 0,45µm 10/pk SDS
| Title |
|---|