574714 Sigma-AldrichSyk Inhibitor IV, BAY 61-3606 - CAS 732983-37-8 - Calbiochem
Syk Inhibitor IV, BAY 61-3606, CAS 732938-37-8, is a cell-permeable, potent, ATP-competitive, reversible, and highly selective inhibitor of Syk tyrosine kinase activity (IC50 = 10 nM).
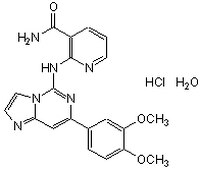
More>> Syk Inhibitor IV, BAY 61-3606, CAS 732938-37-8, is a cell-permeable, potent, ATP-competitive, reversible, and highly selective inhibitor of Syk tyrosine kinase activity (IC50 = 10 nM). Less<<Synonyms: 2-(7-(3,4-Dimethoxyphenyl)-imidazo[1,2-c]pyrimidin-5-ylamino)-nicotinamide, HCl, BAY 61-3606
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 732983-37-8 | C₂₀H₁₈N₆O₃ • HCl • H₂O |
Products
| Catalog Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 574714-2MG | Glass bottle | 2 mg |
| References | |
|---|---|
| References | Yamamoto, N., et al. 2003. J. Pharm. Exp. Ther. 306, 1174. |
| Product Information | |
|---|---|
| CAS number | 732983-37-8 |
| ATP Competitive | Y |
| Form | Yellow solid |
| Hill Formula | C₂₀H₁₈N₆O₃ • HCl • H₂O |
| Chemical formula | C₂₀H₁₈N₆O₃ • HCl • H₂O |
| Hygroscopic | Hygroscopic |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | Syk |
| Primary Target IC<sub>50</sub> | 10 nM |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 574714-2MG | 04055977189698 |
Documentation
Syk Inhibitor IV, BAY 61-3606 - CAS 732983-37-8 - Calbiochem SDS
| Title |
|---|
Syk Inhibitor IV, BAY 61-3606 - CAS 732983-37-8 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 574714 |
References
| Reference overview |
|---|
| Yamamoto, N., et al. 2003. J. Pharm. Exp. Ther. 306, 1174. |
Brochure
| Title |
|---|
| Biologics 33.2 |