681675 Sigma-AldrichWortmannin - CAS 19545-26-7 - Calbiochem
Wortmannin, CAS 19545-26-7, is a cell-permeable, potent, selective, and irreversible inhibitor of PI3-Kinase (IC₅₀ = 5 nM). Does not affect any upstream signaling events.
More>> Wortmannin, CAS 19545-26-7, is a cell-permeable, potent, selective, and irreversible inhibitor of PI3-Kinase (IC₅₀ = 5 nM). Does not affect any upstream signaling events. Less<<Synonyms: KY 12420, MLCK Inhibitor II
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
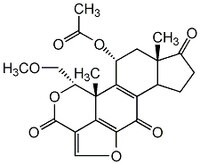
| 19545-26-7 | C₂₃H₂₄O₈ |
Products
| Catalog Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 681675-1MG | Glass bottle | 1 mg |
| Description | |
|---|---|
| Overview | A cell-permeable, fungal metabolite that acts as a potent, selective, and irreversible inhibitor of phosphatidylinositol 3-kinase in purified preparations and cytosolic fractions (IC50 = 5 nM). Blocks the catalytic activity of PI 3-kinase without affecting the upstream signaling events. Preincubation of fibroblasts with wortmannin abolishes PDGF-mediated Ins(3,4,5)P3 formation (IC50 = 5 nM). Also blocks the metabolic effects of insulin in isolated rat adipocytes without affecting the insulin receptor tyrosine kinase activity. Inhibits MAP kinase activation induced by platelet activating factor in guinea pig neutrophils (200 - 300 nM). Also inhibits other kinases such as myosin light chain kinase (IC50 = 200 nM) and PI 4-kinase at concentrations higher than that required for inhibition of PI 3-kinase. Also blocks phospholipase D. A 10 mM (1 mg/233 µl) solution of Wortmannin (Cat. No. 681676) in DMSO is also available. |
| Catalogue Number | 681675 |
| Brand Family | Calbiochem® |
| Synonyms | KY 12420, MLCK Inhibitor II |
| Product Information | |
|---|---|
| CAS number | 19545-26-7 |
| ATP Competitive | N |
| Form | White to off-white solid |
| Hill Formula | C₂₃H₂₄O₈ |
| Chemical formula | C₂₃H₂₄O₈ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | CB9641000 |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 681675-1MG | 04055977225235 |
Documentation
Wortmannin - CAS 19545-26-7 - Calbiochem SDS
| Title |
|---|
Wortmannin - CAS 19545-26-7 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 681675 |
References
| Reference overview |
|---|
| Cross, M.J., et al. 1995. J. Biol. Chem. 270, 25352. Nakamura, I., et al. 1995. FEBS Lett. 361, 79. Ferby, I.M., et al. 1994. J. Biol. Chem. 269, 30485. Okada, T., et al. 1994. J. Biol. Chem. 269, 3568. Wymann, M.P. and Arcaro, A. 1994. Biochem. J. 298, 517. Arcaro, A. and Wymann, M.P. 1993. Biochem. J. 296, 297. Nakanishi, S., et al. 1992. J. Biol. Chem. 267, 2157. Bonser, R.W., et al. 1991. Br. J. Pharmacol. 103, 1237. |
Citations
| Title | |
|---|---|
|
|
| Data Sheet | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|