532813 Sigma-Aldrichp21 Inhibitor, UC2288 - CAS 1394011-91-6 - Calbiochem
A cell-permeable compound that selectively downregulates the expression of p21 (~10 µM), independent of p53.expression, at either transcription or post-transcriptional level.
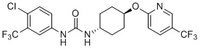
More>> A cell-permeable compound that selectively downregulates the expression of p21 (~10 µM), independent of p53.expression, at either transcription or post-transcriptional level. Less<<Synonyms: 1-(4-Chloro-3-(trifluoromethyl)phenyl)-3-((1r,4r)-4-(5-(trifluoromethyl)pyridin-2-yloxy)cyclohexyl)urea, trans-1-(4-Chloro-3-trifluoromethyl-phenyl)-3-(4-hydroxy-cyclohexyl)-urea, p21/Cip1/CKI/Waf1 Inhibitor, t-CTTU; UC-2288
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 1394011-91-6 | C₂₀H₁₈ClF₆N₃O₂ |
Products
| Catalog Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.32813.0001 | Glass bottle | 10 mg |
| References | |
|---|---|
| References | Gupta, R., et al. 2014. Proc. Natl. Acad. Sci. USA 111, E3062. Wettersten, H.I., et al. 2013. Cancer Biol. Ther. 14, 278. |
| Product Information | |
|---|---|
| CAS number | 1394011-91-6 |
| Form | White solid |
| Hill Formula | C₂₀H₁₈ClF₆N₃O₂ |
| Chemical formula | C₂₀H₁₈ClF₆N₃O₂ |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 5.32813.0001 | 04055977281910 |
Documentation
p21 Inhibitor, UC2288 - CAS 1394011-91-6 - Calbiochem SDS
| Title |
|---|
p21 Inhibitor, UC2288 - CAS 1394011-91-6 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 532813 |
References
| Reference overview |
|---|
| Gupta, R., et al. 2014. Proc. Natl. Acad. Sci. USA 111, E3062. Wettersten, H.I., et al. 2013. Cancer Biol. Ther. 14, 278. |
Technical Info
| Title |
|---|
| Characterization of Estrogen Receptor α Phosphorylation Sites in Breast Cancer Tissue Using the SNAP i.d® 2.0 System |
| White Paper: Further considerations of antibody validation and usage. |
| Data Sheet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|