251010 Sigma-Aldrich1-β-D-Arabinofuranosylcytosine - CAS 147-94-4 - Calbiochem
Anticancer, antiviral agent that is especially effective against leukemias.
More>> Anticancer, antiviral agent that is especially effective against leukemias. Less<<Synonyms: Ara-C, Cytarabine, Cytosine Arabinoside
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
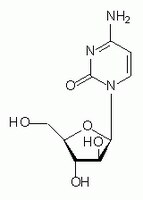
| 147-94-4 | C₉H₁₃N₃O₅ |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 251010-1GM |
|
Plastic ampoule | 1 gm |
|
— |
| Product Information | |
|---|---|
| CAS number | 147-94-4 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₉H₁₃N₃O₅ |
| Chemical formula | C₉H₁₃N₃O₅ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Anticancer, antiviral agent that is especially effective against leukemias |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | HA5425000 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 251010-1GM | 04055977199192 |
Documentation
1-β-D-Arabinofuranosylcytosine - CAS 147-94-4 - Calbiochem SDS
| Title |
|---|
1-β-D-Arabinofuranosylcytosine - CAS 147-94-4 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 251010 |
References
| Reference overview |
|---|
| Zarilli, R., et al. 1999. Gastroenterology 116, 1358. Dessi, F., et al. 1995. J. Neurochem. 64, 1980. Grant, S., et al. 1994. Oncol. Res. 6, 87. Greenberg, A.L., et al. 1994. Cancer 74, 1261. Perrino, F.W., et al. 1997. J. Biol. Chem. 269, 16357. Tomkins, C.E., et al. 1994. J. Cell Sci. 107, 1499. Taddie, J.A., and Traktman, P. 1993. J. Virol. 67, 4323. Brach, M.A., et al. 1992. Mol. Pharmacol. 41, 60. Este, E., et al. 1992. Blood 79, 2246. Owens, J.K., et al. 1992. Cancer Res. 52, 2389. |