128125 Sigma-AldrichAloisine A - CAS 496864-16-5 - Calbiochem
A cell-permeable pyrrolo-pyrazine compound that exerts anti-proliferative effects.
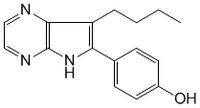
More>> A cell-permeable pyrrolo-pyrazine compound that exerts anti-proliferative effects. Less<<Synonyms: RP107, 7-n-Butyl-6-(4-hydroxyphenyl)[5H]pyrrolo[2,3-b]pyrazine
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 496864-16-5 | C₁₆H₁₇N₃O |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 128125-5MG |
|
Plastic ampoule | 5 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable pyrrolo-pyrazine compound that exerts anti-proliferative effects. Acts as a potent, selective, reversible, and ATP-competitive inhibitor of cyclin-dependent kinases (Cdks; IC50 = 150 nM, 120 nM, 400 nM, and 200 nM for Cdk1/cyclin B, Cdk2/cyclin A, Cdk2/cyclin E, and Cdk5/p25, respectively), glycogen synthase kinase-3 (GSK-3; IC50 = 500 nM and 1.5 µM for GSK-3α, GSK-3β, respectively), and c-Jun N-terminal kinase (JNK; IC50 = ~ 3 - 10 µM). It inhibits several other enzymes (CK1, CK2, MAPKK, PKA, PKG, PKCs, and c-raf) poorly (IC50 ≥ 100 µM). Shown to arrest cells in both G1 and G2 phases (IC50 = 7 µM and 10.5 µM for undifferentiated human teratocarcinoma cells NT2 and differentiated postmitotic neurons hNT, respectively). Shown to selectively stimulate CFTR-dependent iodide efflux in wtCFTR-CHO, Calu-3 and F508del-CFTR-CF15 cells in the presence of 1 µM Forskolin (Cat. No. 344270) with high affinity (EC50 = 150 nM, 140 nM and 111 nM, respectively). |
| Catalogue Number | 128125 |
| Brand Family | Calbiochem® |
| Synonyms | RP107, 7-n-Butyl-6-(4-hydroxyphenyl)[5H]pyrrolo[2,3-b]pyrazine |
| References | |
|---|---|
| References | Noel, S., et al. 2006. J. Pharm. Exp. Ther. , 319, 349. Mettey, Y., et al. 2003. J. Med. Chem. 46, 222. |
| Product Information | |
|---|---|
| CAS number | 496864-16-5 |
| ATP Competitive | Y |
| Form | Yellow solid |
| Hill Formula | C₁₆H₁₇N₃O |
| Chemical formula | C₁₆H₁₇N₃O |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 128125-5MG | 04055977222210 |
Documentation
Aloisine A - CAS 496864-16-5 - Calbiochem SDS
| Title |
|---|
Aloisine A - CAS 496864-16-5 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 128125 |
References
| Reference overview |
|---|
| Noel, S., et al. 2006. J. Pharm. Exp. Ther. , 319, 349. Mettey, Y., et al. 2003. J. Med. Chem. 46, 222. |
| Data Sheet | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|