Protein crystals in Adenovirus type 5-infected cells: requirements for intranuclear crystallogenesis, structural and functional analysis.
Franqueville, L; Henning, P; Magnusson, M; Vigne, E; Schoehn, G; Blair-Zajdel, ME; Habib, N; Lindholm, L; Blair, GE; Hong, SS; Boulanger, P
PloS one
3
e2894
2008
Show Abstract
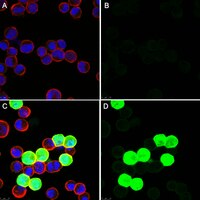
Intranuclear crystalline inclusions have been observed in the nucleus of epithelial cells infected with Adenovirus serotype 5 (Ad5) at late steps of the virus life cycle. Using immuno-electron microscopy and confocal microscopy of cells infected with various Ad5 recombinants modified in their penton base or fiber domains, we found that these inclusions represented crystals of penton capsomers, the heteromeric capsid protein formed of penton base and fiber subunits. The occurrence of protein crystals within the nucleus of infected cells required the integrity of the fiber knob and part of the shaft domain. In the knob domain, the region overlapping residues 489-492 in the FG loop was found to be essential for crystal formation. In the shaft, a large deletion of repeats 4 to 16 had no detrimental effect on crystal inclusions, whereas deletion of repeats 8 to 21 abolished crystal formation without altering the level of fiber protein expression. This suggested a crucial role of the five penultimate repeats in the crystallisation process. Chimeric pentons made of Ad5 penton base and fiber domains from different serotypes were analyzed with respect to crystal formation. No crystal was found when fiber consisted of shaft (S) from Ad5 and knob (K) from Ad3 (heterotypic S5-K3 fiber), but occurred with homotypic S3K3 fiber. However, less regular crystals were observed with homotypic S35-K35 fiber. TB5, a monoclonal antibody directed against the Ad5 fiber knob was found by immunofluorescence microscopy to react with high efficiency with the intranuclear protein crystals in situ. Data obtained with Ad fiber mutants indicated that the absence of crystalline inclusions correlated with a lower infectivity and/or lower yields of virus progeny, suggesting that the protein crystals might be involved in virion assembly. Thus, we propose that TB5 staining of Ad-infected 293 cells can be used as a prognostic assay for the viability and productivity of fiber-modified Ad5 vectors. | 18682854
 |
Human cytomegalovirus IE86 attenuates virus- and tumor necrosis factor alpha-induced NFkappaB-dependent gene expression.
Taylor, RT; Bresnahan, WA
Journal of virology
80
10763-71
2006
Show Abstract
Human cytomegalovirus (HCMV) infection regulates a number of genes involved in the host antiviral response. We have previously reported that HCMV attenuates the expression of beta interferon (IFN-beta) and a number of proinflammatory chemokines, and this attenuation is mediated by the HCMV immediate-early protein IE86. The present study seeks to identify the mechanism by which IE86 blocks IFN-beta expression. We demonstrate that the induction of IFN-beta during HCMV infection requires the activation of both the IRF-3 and the NFkappaB pathways. Therefore, IE86 may target either pathway to block IFN-beta expression. Our results show that IE86 does not block IRF-3 phosphorylation, dimerization, nuclear translocation, or target gene expression. However, using gel shift analysis, we demonstrate that IE86 efficiently inhibits virus-induced binding of NFkappaB to the IFN-beta promoter, resulting in attenuation of IFN-beta and NFkappaB-dependent gene expression. Furthermore, IE86 expression inhibits tumor necrosis factor alpha-induced NFkappaB DNA binding and target gene expression. Together, these results identify IE86 as a NFkappaB antagonist, which results in the suppression of NFkappaB-dependent cytokine and chemokine gene expression. Full Text Article | 17041226
 |