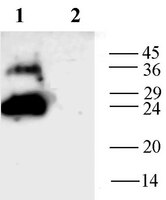
Chronic ingestion of high dosed Phikud Navakot extraction induces mesangiolysis in rats with alteration of AQP1 and Hsp60 expressions.
Kengkoom, K; Ampawong, S
BioMed research international
2015
462387
2015
Show Abstract
Phikud Navakot (PN) is commonly used in Thai traditional medicine for alleviation of cardiovascular and cerebrovascular symptoms; however little is known about the chronic toxicity effects of the extracts from the herbs in PN. Repeated extraction doses of 10, 100, and 1,000 mg/kg/day were randomly administered to both male and female Sprague Dawley rats for 12 months. Histopathological study revealed that mesangiolysis was predominately found at the highest dose. Aquaporin 1 (AQP1) expression in the mesangiolytic glomeruli was significantly lower than in the intact glomeruli. This may be relevant to an imbalance of vascular function manifested by AQP1 alteration. In the mesangiolytic glomeruli, 60 kDa heat shock protein (Hsp60) was significantly upregulated on the endothelial lining cells of aneurysm and vascular cyst. Hsp60 increase may be related to endothelial cell damage due to its intracellular protective role. Blood urea nitrogen and creatinine levels remained within their normal range indicating well-functioning renal reserve function. In conclusion, high dosed PN may affect the endothelium leading to inability of vascular permeability and consequence to mesangiolysis. Our results suggest that only a high dose of chronic oral administration of PN is relatively toxic in association with mesangiolysis. The NOAEL was determined to be 100 mg/kg/day. | Immunohistochemistry | | 25815318
 |
Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis.
Misu, T, et al.
Brain, 130: 1224-34 (2007)
2007
Show Abstract
Neuromyelitis optica (NMO) is an inflammatory and necrotizing disease clinically characterized by selective involvement of the optic nerves and spinal cord. There has been a long controversy as to whether NMO is a variant of multiple sclerosis (MS) or a distinct disease. Recently, an NMO-specific antibody (NMO-IgG) was found in the sera from patients with NMO, and its target antigen was identified as aquaporin 4 (AQP4) water channel protein, mainly expressed in astroglial foot processes. However, the pathogenetic role of the AQP4 in NMO remains unknown. We did an immunohistopathological study on the distribution of AQP4, glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), activated complement C9neo and immunoglobulins in the spinal cord lesions and medulla oblongata of NMO (n = 12), MS (n = 6), brain and spinal infarction (n = 7) and normal control (n = 8). The most striking finding was that AQP4 immunoreactivity was lost in 60 out of a total of 67 acute and chronic NMO lesions (90%), but not in MS plaques. The extensive loss of AQP4 accompanied by decreased GFAP staining was evident, especially in the active perivascular lesions, where immunoglobulins and activated complements were deposited. Interestingly, in those NMO lesions, MBP-stained myelinated fibres were relatively preserved despite the loss of AQP4 and GFAP staining. The areas surrounding the lesions in NMO had enhanced expression of AQP4 and GFAP, which reflected reactive gliosis. In contrast, AQP4 immunoreactivity was well preserved and rather strongly stained in the demyelinating MS plaques, and infarcts were also stained for AQP4 from the very acute phase of necrosis to the chronic stage of astrogliosis. In normal controls, AQP4 was diffusely expressed in the entire tissue sections, but the staining in the spinal cord was stronger in the central grey matter than in the white matter. The present study demonstrated that the immunoreactivities of AQP4 and GFAP were consistently lost from the early stage of the lesions in NMO, notably in the perivascular regions with complement and immunoglobulin deposition. These features in NMO were distinct from those of MS and infarction as well as normal controls, and suggest that astrocytic impairment associated with the loss of AQP4 and humoral immunity may be important in the pathogenesis of NMO lesions. | Immunohistochemistry (Paraffin) | Human | 17405762
 |
Aquaporins in complex tissues. I. Developmental patterns in respiratory and glandular tissues of rat.
King, L S, et al.
Am. J. Physiol., 273: C1541-8 (1997)
1997
Show Abstract
Developmental expression of aquaporin water transport proteins is not well understood in respiratory tract or secretory glands; here we define aquaporin protein ontogeny in rat. Expression of aquaporin-3 (AQP3), AQP4, and AQP5 proteins occurs within 2 wk after birth, whereas AQP1 first appears before birth. In most tissues, aquaporin protein expression increases progressively, although transient high-level expression is noted in distal lung (AQP4 at postnatal day +2) and trachea (AQP5 at postnatal day +21 and AQP3 at postnatal day +42). In mature animals, AQP5 is abundant in distal lung and salivary glands, AQP3 and AQP4 are present in trachea, and AQP1 is present in all of these tissues except salivary glands. Surprisingly, all four aquaporin proteins are highly abundant in nasopharynx. Unlike AQP1, corticosteroids did not induce expression of AQP3, AQP4, or AQP5 in lung. Our results seemingly implicate aquaporins in proximal airway humidification, glandular secretion, and perinatal clearance of fluid from distal airways. However, the studies underscore a need for detailed immunohistochemical characterizations and definitive functional studies. | | | 9374639
 |
Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat.
Nielsen, S, et al.
Am. J. Physiol., 273: C1549-61 (1997)
1997
Show Abstract
The molecular pathways for fluid transport in pulmonary, oral, and nasal tissues are still unresolved. Here we use immunocytochemistry and immunoelectron microscopy to define the sites of expression of four aquaporins in the respiratory tract and glandular epithelia, where they reside in distinct, nonoverlapping sites. Aquaporin-1 (AQP1) is present in apical and basolateral membranes of bronchial, tracheal, and nasopharyngeal vascular endothelium and fibroblasts. AQP5 is localized to the apical plasma membrane of type I pneumocytes and the apical plasma membranes of secretory epithelium in upper airway and salivary glands. In contrast, AQP3 is present in basal cells of tracheal and nasopharyngeal epithelium and is abundant in basolateral membranes of surface epithelial cells of nasal conchus. AQP4 resides in basolateral membranes of columnar cells of bronchial, tracheal, and nasopharyngeal epithelium; in nasal conchus AQP4 is restricted to basolateral membranes of a subset of intra- and subepithelial glands. These sites of expression suggest that transalveolar water movement, modulation of airway surface liquid, air humidification, and generation of nasopharyngeal secretions involve a coordinated network of aquaporin water channels. | | | 9374640
 |
Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family.
Preston, G M and Agre, P
Proc. Natl. Acad. Sci. U.S.A., 88: 11110-4 (1991)
1991
Show Abstract
CHIP28 is a 28-kDa integral membrane protein with similarities to membrane channels and is found in erythrocytes and renal tubules. A cDNA for CHIP28 was isolated from human fetal liver cDNA template by a three-step polymerase chain reaction (PCR) cloning strategy, starting with degenerate oligonucleotide primers corresponding to the N-terminal amino acid sequence determined from purified CHIP28 protein. Using the third-step PCR product as a probe, we isolated a recombinant from a human bone marrow cDNA library. The combined sequence of the PCR products and bone marrow cDNA contains 38 base pairs of 5' untranslated nucleotide sequence, an 807-bp open reading frame, and approximately 2 kilobases of 3' untranslated sequence containing a polyadenylation signal. This corresponds to the 3.1-kilobase transcript identified by RNA blot-hybridization analysis. Authenticity of the deduced amino acid sequence of the CHIP28 protein C terminus was confirmed by expression and immunoblotting. Analysis of the deduced amino acid sequence suggests that CHIP28 protein contains six bilayer-spanning domains, two exofacial potential N-glycosylation sites, and intracellular N and C termini. Search of the DNA sequence data base revealed a strong homology with the major intrinsic protein of bovine lens, which is the prototype of an ancient but recently recognized family of membrane channels. These proteins are believed to form channels permeable to water and possibly other small molecules. CHIP28 shares homology with all known members of this channel family, and it is speculated that CHIP28 has a similar function. | | | 1722319
 |