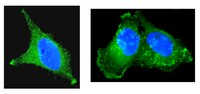
Hax-1 is required for Rac1-Cortactin interaction and ovarian carcinoma cell migration.
Gomathinayagam, R; Muralidharan, J; Ha, JH; Varadarajalu, L; Dhanasekaran, DN
Genes & cancer
5
84-99
2014
Show Abstract
Hax-1 is a multifunctional protein, which is involved in diverse cellular signaling pathways including tumor cell survival and migration. We have shown previously that cell migration stimulated by the oncogenic G protein, G13, requires Hax-1 for the formation of a functional complex involving Gα13, Rac1, and cortactin. However, the role of Hax-1 in cancer cell migration or its role in Rac1-cortactin complex formation, which is known to be required for such migration remains to be characterized. Results focused on resolving the role of Hax-1 in ovarian cancer pathophysiology indicate that Hax-1 is overexpressed in ovarian cancer cells and the silencing of Hax-1 inhibits lysophosphatidic acid (LPA)- or fetal bovine serum-stimulated migration of these cells. In addition, silencing of Hax-1 greatly reduces Rac1-cortactin interaction and their colocalization in SKOV3 cells. Mapping the structural domains of Hax-1 indicates that it interacts with cortactin via domains spanning amino acids 1 to 56 (Hax-D1) and amino acids 113 to 168 (Hax-D3). Much weaker interaction with cortactin was also observed with the region of Hax-1 spanning amino acids 169 - 224 (Hax-D4). Similar mapping of Hax-1 domains involved in Rac1 interaction indicates that it associates with Rac1 via two primary domains spanning amino acids 57 to 112 (Hax-D2) and 169 to 224 (Hax-D4). Furthermore, expression of either of these domains inhibits LPA-mediated migration of SKOV3 cells, possibly through their ability to exert competitive inhibition on endogenous Hax-1-Rac1 and/or Hax-1-cortactin interaction. More significantly, expression of Hax-D4 drastically reduces Rac1-cortactin colocalization in SKOV3 cells along with an attenuation of LPA-stimulated migration. Thus our results presented here describe for the first time that Hax-1 interaction is required for the association between Rac1 and cortactin and that these multiple interactions are required for the LPA-stimulated migration of SKOV3 ovarian cancer cells. | | 25053987
 |
Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control.
Lua, Bee Leng and Low, Boon Chuan
FEBS Lett., 579: 577-85 (2005)
2005
Show Abstract
Cortactin is an important molecular scaffold for actin assembly and organization. Novel mechanistic functions of cortactin have emerged with more interacting partners identified, revealing its multifaceted roles in regulating actin cytoskeletal networks that are necessary for endocytosis, cell migration and invasion, adhesion, synaptic organization and cell morphogenesis. These processes are mediated by its multi-domains binding to F-actin and Arp2/3 complex and various SH3 targets. Furthermore, its role in actin remodeling is subjected to regulation by tyrosine and serine/threonine kinases. Elucidating the mechanisms underlying cortactin phosphorylation and its functional consequences would provide new insights to various aspects of cell dynamics control. | | 15670811
 |
Actions by actin: reciprocal regulation of cortactin activity by tyrosine kinases and F-actin.
Gunst, Susan J
Biochem. J., 380: e7-8 (2004)
2004
Show Abstract
The polymerization of actin is catalysed by the Arp (actin-related protein) 2/3 complex, which acts downstream of a variety of receptors and signalling cascades. Intermediary molecules such as cortactin bind to the Arp2/3 complex and stimulate its activity, thus promoting actin polymerization and actin filament stabilization. New data in this issue of the Biochemical Journal by the Kapus group suggest that cortactin is reciprocally regulated by filamentous (F) actin and tyrosine kinases. This suggests a new paradigm for considering the cellular processes that regulate the dynamic organization of the actin cytoskeleton. | | 15154835
 |
Cortactin signalling and dynamic actin networks.
Daly, Roger J
Biochem. J., 382: 13-25 (2004)
2004
Show Abstract
Cortactin was first identified over a decade ago, and its initial characterization as both an F-actin binding protein and v-Src substrate suggested that it was likely to be a key regulator of actin rearrangements in response to tyrosine kinase signalling. The recent discovery that cortactin binds and activates the actin related protein (Arp)2/3 complex, and thus regulates the formation of branched actin networks, together with the identification of multiple protein targets of the cortactin SH3 domain, have revealed diverse cellular roles for this protein. This article reviews current knowledge regarding the role of cortactin in signalling to the actin cytoskeleton in the context of these developments. | | 15186216
 |
Cortactin: coupling membrane dynamics to cortical actin assembly.
Weed, S A and Parsons, J T
Oncogene, 20: 6418-34 (2001)
2001
Show Abstract
Exposure of cells to a variety of external signals causes rapid changes in plasma membrane morphology. Plasma membrane dynamics, including membrane ruffle and microspike formation, fusion or fission of intracellular vesicles, and the spatial organization of transmembrane proteins, is directly controlled by the dynamic reorganization of the underlying actin cytoskeleton. Two members of the Rho family of small GTPases, Cdc42 and Rac, have been well established as mediators of extracellular signaling events that impact cortical actin organization. Actin-based signaling through Cdc42 and Rac ultimately results in activation of the actin-related protein (Arp) 2/3 complex, which promotes the formation of branched actin networks. In addition, the activity of both receptor and non-receptor protein tyrosine kinases along with numerous actin binding proteins works in concert with Arp2/3-mediated actin polymerization in regulating the formation of dynamic cortical actin-associated structures. In this review we discuss the structure and role of the cortical actin binding protein cortactin in Rho GTPase and tyrosine kinase signaling events, with the emphasis on the roles cortactin plays in tyrosine phosphorylation-based signal transduction, regulating cortical actin assembly, transmembrane receptor organization and membrane dynamics. We also consider how aberrant regulation of cortactin levels contributes to tumor cell invasion and metastasis. | | 11607842
 |
Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape
M A McNiven 1 , L Kim, E W Krueger, J D Orth, H Cao, T W Wong
J Cell Biol
151(1)
187-98
2000
Show Abstract
The dynamin family of large GTPases has been implicated in the formation of nascent vesicles in both the endocytic and secretory pathways. It is believed that dynamin interacts with a variety of cellular proteins to constrict membranes. The actin cytoskeleton has also been implicated in altering membrane shape and form during cell migration, endocytosis, and secretion and has been postulated to work synergistically with dynamin and coat proteins in several of these important processes. We have observed that the cytoplasmic distribution of dynamin changes dramatically in fibroblasts that have been stimulated to undergo migration with a motagen/hormone. In quiescent cells, dynamin 2 (Dyn 2) associates predominantly with clathrin-coated vesicles at the plasma membrane and the Golgi apparatus. Upon treatment with PDGF to induce cell migration, dynamin becomes markedly associated with membrane ruffles and lamellipodia. Biochemical and morphological studies using antibodies and GFP-tagged dynamin demonstrate an interaction with cortactin. Cortactin is an actin-binding protein that contains a well defined SH3 domain. Using a variety of biochemical methods we demonstrate that the cortactin-SH3 domain associates with the proline-rich domain (PRD) of dynamin. Functional studies that express wild-type and mutant forms of dynamin and/or cortactin in living cells support these in vitro observations and demonstrate that an increased expression of cortactin leads to a significant recruitment of endogenous or expressed dynamin into the cell ruffle. Further, expression of a cortactin protein lacking the interactive SH3 domain (CortDeltaSH3) significantly reduces dynamin localization to the ruffle. Accordingly, transfected cells expressing Dyn 2 lacking the PRD (Dyn 2(aa)DeltaPRD) sequester little of this protein to the cortactin-rich ruffle. Interestingly, these mutant cells are viable, but display dramatic alterations in morphology. This change in shape appears to be due, in part, to a striking increase in the number of actin stress fibers. These findings provide the first demonstration that dynamin can interact with the actin cytoskeleton to regulate actin reorganization and subsequently cell shape. | Immunoprecipitation | 11018064
 |
Adenovirus E4 open reading frame 4-induced apoptosis involves dysregulation of Src family kinases
J N Lavoie 1 , C Champagne, M C Gingras, A Robert
J Cell Biol
150(5)
1037-56
2000
Show Abstract
The adenoviral early region 4 open reading frame 4 (E4orf4) death factor induces p53-independent apoptosis in many cell types and appears to kill selectively transformed cells. Here we show that expression of E4orf4 in transformed epithelial cells results in early caspase-independent membrane blebbing, associated with changes in the organization of focal adhesions and actin cytoskeleton. Evidence that E4orf4 can associate with and modulate Src family kinase activity, inhibiting Src-dependent phosphorylation of focal adhesion kinase (FAK) and paxillin while increasing phosphorylation of cortactin and some other cellular proteins, is presented. Furthermore, E4orf4 dramatically inhibited the ability of FAK and c-src to cooperate in induction of tyrosine phosphorylation of cellular substrates, suggesting that E4orf4 can interfere with the formation of a signaling complex at focal adhesion sites. Consistent with a functional role for E4orf4-Src interaction, overexpression of activated c-src dramatically potentiated E4orf4-induced membrane blebbing and apoptosis, whereas kinase dead c-src constructs inhibited E4orf4 effects on cell morphology and death. Moreover treatment of E4orf4-expressing cells with PP2, a selective Src kinase inhibitor, led to inhibition of E4orf4-dependent membrane blebbing and later to a marked decrease in E4orf4-induced nuclear condensation. Taken together, these observations indicate that expression of adenovirus 2 E4orf4 can initiate caspase-independent extranuclear manifestations of apoptosis through a modulation of Src family kinases and that these are involved in signaling E4orf4-dependent apoptosis. This study also suggests that Src family kinases are likely to play a role in the cytoplasmic execution of apoptotic programs. | Immunoprecipitation | 10973994
 |
Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse
T Katsube 1 , M Takahisa, R Ueda, N Hashimoto, M Kobayashi, S Togashi
J Biol Chem
273(45)
29672-7
1998
Show Abstract
Cortactin is an actin filament-binding protein localizing at cortical regions of cells and a prominent substrate for Src family protein-tyrosine kinases in response to multiple extracellular stimuli. Human cortactin has been identified as a protein product of a putative oncogene, EMS1. In this report, we describe the identification of a Drosophila homolog of cortactin as a molecule that interacts with Drosophila ZO-1 using yeast two-hybrid screening. Drosophila cortactin is a 559-amino acid protein highly expressed in embryos, larvae, and pupae but relatively underexpressed in adult flies. Deletion and substitution mutant analyses revealed that the SH3 domain of Drosophila cortactin binds to a PXXP motif in the proline-rich domain of Drosophila ZO-1. Colocalization of these proteins at cell-cell junction sites was evident under a confocal laser-scanning microscope. In vivo association was confirmed by coimmunoprecipitation of cortactin and ZO-1 from Drosophila embryo lysates. We also demonstrate an association for each of the murine homologs by immunoprecipitation analyses of mouse tissue lysates. Our previous work has demonstrated the involvement of ZO-1 in a signaling pathway that regulates expression of the emc gene in Drosophila. The potential roles of the cortactin.ZO-1 complex in cell adhesion and cell signaling are discussed. | Immunoblotting (Western) | 9792678
 |
Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER
L Kim 1 , T W Wong
J Biol Chem
273(36)
23542-8
1998
Show Abstract
Previous characterization of the nonreceptor tyrosine kinase FER identified a tight physical association with the catenin pp120 and led to the suggestion that FER may be involved in cell-cell signaling. To further understand the function of FER, we have continued our analyses of the interaction of FER with pp120 and other proteins. The majority of FER is localized to the cytoplasmic fraction where it forms a complex with the actin-binding protein cortactin. The Src homology 2 sequence of FER is required for directly binding cortactin, and phosphorylation of the FER-cortactin complex is up-regulated in cells treated with peptide growth factors. Using a dominant-negative mutant of FER, we provided evidence that FER kinase activity is required for the growth factor-dependent phosphorylation of cortactin. These data suggest that cortactin is likely to be a direct substrate of FER. Our observations provide additional support for a role of FER in mediating signaling from the cell surface, via growth factor receptors, to the cytoskeleton. The nature of the FER-cortactin interaction, and their putative enzyme-substrate relationship, support the previous proposal that one of the functions of the Src homology 2 sequences of nonreceptor tyrosine kinases is to provide a binding site for their preferred substrates. | Immunoblotting (Western), Immunofluorescence | 9722593
 |
Mechanical strain induces pp60src activation and translocation to cytoskeleton in fetal rat lung cells.
Liu, M, et al.
J. Biol. Chem., 271: 7066-71 (1996)
1996
Show Abstract
We have previously shown that mechanical strain-induced fetal rat lung cell proliferation is transduced via the phospholipase C-gamma-protein kinase C pathway. In the present study, we found that protein-tyrosine kinase activity of fetal lung cells increased after a short period of strain, which was accompanied by tyrosine phosphorylation of proteins of approximately 110-130 kDa. Several components of this complex were identified as pp60srcsubstrates. Strain increased pp60src activity in the cytoskeletal fraction, which coincided with a shift in subcellular distribution of pp60src from the Triton-soluble to the cytoskeletal fraction. Strain-induced pp60src translocation did not appear to be mediated via the focal adhesion kinase-paxillin pathway. In contrast, strain increased the association between pp60src and the actin filament-associated protein of 110 kDa. Preincubation of cells with herbimycin A, a tyrosine kinase inhibitor, abolished strain-induced phospholipase C-gamma1 tyrosine phosphorylation and its coimmunoprecipitation with pp60src. It also inhibited strain-induced DNA synthesis. These results suggest that activation of pp60src is an upstream event of the phospholipase C-gamma-protein kinase C pathway that may represent an important mechanism by which mechanical perturbations are converted to biological reactions in fetal lung cells. | Immunoblotting (Western) | 8636139
 |