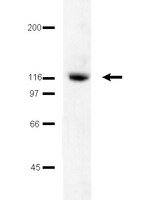
FAK activity protects nucleostemin in facilitating breast cancer spheroid and tumor growth.
Tancioni, I; Miller, NL; Uryu, S; Lawson, C; Jean, C; Chen, XL; Kleinschmidt, EG; Schlaepfer, DD
Breast cancer research : BCR
17
47
2015
Show Abstract
Focal adhesion kinase (FAK) controls cell growth and survival downstream of integrin-matrix receptors. Upon adhesion loss or FAK inhibition, FAK can translocate to the nucleus. The nucleolus is a non-membrane nuclear structure that regulates ribosome biogenesis and cell proliferation. Nucleostemin (NS), a nucleolar-localized protein, modulates cell cycle progression, stemness, and three-dimensional tumor spheroid formation. The signaling pathways that regulate NS levels in tumors remain undefined.Human breast carcinoma cells were evaluated for growth in culture (adherent and anchorage-independent spheroid) and as orthotopic tumors. FAK signaling was evaluated by pharmacological FAK inhibitor addition (PF-271, IC50~0.1 μM) and by small hairpin RNA (shRNA) knockdown followed by re-expression of FAK wildtype (WT) or a kinase-dead (KD, K454R) FAK point mutant. Immunoblotting was used to evaluate FAK, NS, nucleolar phosphoprotein B23, and nucleolin levels. Total and phosphospecific antibody imunoblotting were used to detect changes in FAK, Akt kinase (Akt also known as protein kinase B), and 4E-binding protein 1 (4E-BP1) phosphorylation, a translation repressor protein and target of the mammalian target of rapamycin (mTOR) complex. Immunohistochemical, co-immunoprecipitation, and cellular fractionation analyses were used to evaluate FAK association with nucleoli.Pharmacological (0.1 μM PF-271) or genetic inhibition of FAK activity prevents MDA-MB-231 and 4T1L breast carcinoma growth as spheroids and as orthotopic tumors. FAK inhibition triggers proteasome-mediated decreased NS levels but no changes in other nucleolar proteins such as B23 (nucleophosmin) or nucleolin. Active FAK was associated with purified nucleoli of anchorage-independent cells and present within nucleoli of human invasive ductal carcinoma tumor samples. FAK co-immunoprecipitated with B23 that binds NS and a complex between FAK, NS, Akt, and mTOR was detected. Constitutively-active Akt kinase promoted tumor spheroid growth, stabilized NS levels, and promoted pS65 4E-BP1 phosphorylation in the presence of inhibited FAK. Rapamycin lowered NS levels and inhibited pS65 4E-BP1 phosphorylation in cells with activated Akt-mTOR signaling.FAK signaling occurs in the nucleolus, active FAK protects NS, and Akt-mTOR pathway regulates NS protein stability needed for breast carcinoma spheroid and tumor growth. | | | 25880415
 |
Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model.
Sidhu, H; Dansie, LE; Hickmott, PW; Ethell, DW; Ethell, IM
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
9867-79
2014
Show Abstract
Fmr1 knock-out (ko) mice display key features of fragile X syndrome (FXS), including delayed dendritic spine maturation and FXS-associated behaviors, such as poor socialization, obsessive-compulsive behavior, and hyperactivity. Here we provide conclusive evidence that matrix metalloproteinase-9 (MMP-9) is necessary to the development of FXS-associated defects in Fmr1 ko mice. Genetic disruption of Mmp-9 rescued key aspects of Fmr1 deficiency, including dendritic spine abnormalities, abnormal mGluR5-dependent LTD, as well as aberrant behaviors in open field and social novelty tests. Remarkably, MMP-9 deficiency also corrected non-neural features of Fmr1 deficiency-specifically macroorchidism-indicating that MMP-9 dysregulation contributes to FXS-associated abnormalities outside the CNS. Further, MMP-9 deficiency suppressed elevations of Akt, mammalian target of rapamycin, and eukaryotic translation initiation factor 4E phosphorylation seen in Fmr1 ko mice, which are also associated with other autistic spectrum disorders. These findings establish that MMP-9 is critical to the mechanisms responsible for neural and non-neural aspects of the FXS phenotype. | Immunofluorescence | | 25057190
 |
N-wasp is required for structural integrity of the blood-testis barrier.
Xiao, X; Mruk, DD; Tang, EI; Massarwa, R; Mok, KW; Li, N; Wong, CK; Lee, WM; Snapper, SB; Shilo, BZ; Schejter, ED; Cheng, CY
PLoS genetics
10
e1004447
2014
Show Abstract
During spermatogenesis, the blood-testis barrier (BTB) segregates the adluminal (apical) and basal compartments in the seminiferous epithelium, thereby creating a privileged adluminal environment that allows post-meiotic spermatid development to proceed without interference of the host immune system. A key feature of the BTB is its continuous remodeling within the Sertoli cells, the major somatic component of the seminiferous epithelium. This remodeling is necessary to allow the transport of germ cells towards the seminiferous tubule interior, while maintaining intact barrier properties. Here we demonstrate that the actin nucleation promoting factor Neuronal Wiskott-Aldrich Syndrome Protein (N-WASP) provides an essential function necessary for BTB restructuring, and for maintaining spermatogenesis. Our data suggests that the N-WASP-Arp2/3 actin polymerization machinery generates branched-actin arrays at an advanced stage of BTB remodeling. These arrays are proposed to mediate the restructuring process through endocytic recycling of BTB components. Disruption of N-WASP in Sertoli cells results in major structural abnormalities to the BTB, including mis-localization of critical junctional and cytoskeletal elements, and leads to disruption of barrier function. These impairments result in a complete arrest of spermatogenesis, underscoring the critical involvement of the somatic compartment of the seminiferous tubules in germ cell maturation. | | | 24967734
 |
Absence of γ-sarcoglycan alters the response of p70S6 kinase to mechanical perturbation in murine skeletal muscle.
Moorwood, C; Philippou, A; Spinazzola, J; Keyser, B; Macarak, EJ; Barton, ER
Skeletal muscle
4
13
2014
Show Abstract
The dystrophin glycoprotein complex (DGC) is located at the sarcolemma of muscle fibers, providing structural integrity. Mutations in and loss of DGC proteins cause a spectrum of muscular dystrophies. When only the sarcoglycan subcomplex is absent, muscles display severe myofiber degeneration, but little susceptibility to contractile damage, suggesting that disease occurs not by structural deficits but through aberrant signaling, namely, loss of normal mechanotransduction signaling through the sarcoglycan complex. We extended our previous studies on mechanosensitive, γ-sarcoglycan-dependent ERK1/2 phosphorylation, to determine whether additional pathways are altered with the loss of γ-sarcoglycan.We examined mechanotransduction in the presence and absence of γ-sarcoglycan, using C2C12 myotubes, and primary cultures and isolated muscles from C57Bl/6 (C57) and γ-sarcoglycan-null (γ-SG(-/-)) mice. All were subjected to cyclic passive stretch. Signaling protein phosphorylation was determined by immunoblotting of lysates from stretched and non-stretched samples. Calcium dependence was assessed by maintaining muscles in calcium-free or tetracaine-supplemented Ringer's solution. Dependence on mTOR was determined by stretching isolated muscles in the presence or absence of rapamycin.C2C12 myotube stretch caused a robust increase in P-p70S6K, but decreased P-FAK and P-ERK2. Neither Akt nor ERK1 were responsive to passive stretch. Similar but non-significant trends were observed in C57 primary cultures in response to stretch, and γ-SG(-/-) cultures displayed no p70S6K response. In contrast, in isolated muscles, p70S6K was mechanically responsive. Basal p70S6K activation was elevated in muscles of γ-SG(-/-) mice, in a calcium-independent manner. p70S6K activation increased with stretch in both C57 and γ-SG(-/-) isolated muscles, and was sustained in γ-SG(-/-) muscles, unlike the transient response in C57 muscles. Rapamycin treatment blocked all of p70S6K activation in stretched C57 muscles, and reduced downstream S6RP phosphorylation. However, even though rapamycin treatment decreased p70S6K activation in stretched γ-SG(-/-) muscles, S6RP phosphorylation remained elevated.p70S6K is an important component of γ-sarcoglycan-dependent mechanotransduction in skeletal muscle. Our results suggest that loss of γ-sarcoglycan uncouples the response of p70S6K to stretch and implies that γ-sarcoglycan is important for inactivation of this pathway. Overall, we assert that altered load-sensing mechanisms exist in muscular dystrophies where the sarcoglycans are absent. | | | 25024843
 |
Intracellular modifiers of integrin alpha 6p production in aggressive prostate and breast cancer cell lines.
Kacsinta, AD; Rubenstein, CS; Sroka, IC; Pawar, S; Gard, JM; Nagle, RB; Cress, AE
Biochemical and biophysical research communications
454
335-40
2014
Show Abstract
Cancer metastasis is a multi-step process in which tumor cells gain the ability to invade beyond the primary tumor and colonize distant sites. The mechanisms regulating the metastatic process confer changes to cell adhesion receptors including the integrin family of receptors. Our group previously discovered that the α6 integrin (ITGA6/CD49f) is post translationally modified by urokinase plasminogen activator (uPA) and its receptor, urokinase plasminogen activator receptor (uPAR), to form the variant ITGA6p. This variant of ITGA6 is a cleaved form of the receptor that lacks the ligand-binding domain. Although it is established that the uPA/uPAR axis drives ITGA6 cleavage, the mechanisms regulating cleavage have not been defined. Intracellular integrin dependent "inside-out" signaling is a major regulator of integrin function and the uPA/uPAR axis. We hypothesized that intracellular signaling molecules play a role in formation of ITGA6p to promote cell migration during cancer metastasis. In order to test our hypothesis, DU145 and PC3B1 prostate cancer and MDA-MB-231 breast cancer cell lines were treated with small interfering RNA targeting actin and the intracellular signaling regulators focal adhesion kinase (FAK), integrin linked kinase (ILK), and paxillin. The results demonstrated that inhibition of actin, FAK, and ILK expression resulted in significantly increased uPAR expression and ITGA6p production. Inhibition of actin increased ITGA6p, although inhibition of paxillin did not affect ITGA6p formation. Taken together, these results suggest that FAK and ILK dependent "inside-out" signaling, and actin dynamics regulate extracellular production of ITGA6p and the aggressive phenotype. | | | 25450398
 |
p-FAK-Tyr(397) regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization.
Wan, HT; Mruk, DD; Li, SY; Mok, KW; Lee, WM; Wong, CK; Cheng, CY
American journal of physiology. Endocrinology and metabolism
305
E687-99
2013
Show Abstract
During spermatogenesis, the molecular mechanism that confers spermatid adhesion to the Sertoli cell at the apical ectoplasmic specialization (apical ES), a testis-specific F-actin-rich adherens junction, in the rat testis remains elusive. Herein, the activated form of focal adhesion kinase (FAK), p-FAK-Tyr(397), a component of the apical ES that was expressed predominantly and stage specifically in stage VII-early stage VIII tubules, was found to be a crucial apical ES regulator. Using an FAK-Y397E phosphomimetic mutant cloned in a mammalian expression vector for its transfection vs. FAK and vector alone in adult rat testes in vivo, its overexpression was found to cause defects in spermiation. These defects in spermiation were manifested by entrapment of spermatids in the seminiferous epithelium in late stage VIII-X tubules and were mediated by a disruption on the spatiotemporal expression and/or mislocalization of actin regulatory protein actin-related protein 3, which induces branched actin polymerization, epidermal growth factor receptor pathway substrate 8 (an actin barbed end capping and bundling protein), and palladin (an actin cross-linking and bundling protein). This thus perturbed changes of F-actin organization at the apical ES to facilitate spermiation, which also led to a concomitant alteration in the distribution and upregulation of adhesion proteins nectin-2 and nectin-3 at the apical ES. As such, nectin-2 and -3 remained at the apical ES to anchor step 19 spermatids on to the epithelium, delaying spermiation. These findings illustrate a mechanistic pathway mediated by p-FAK-Tyr(397) that regulates spermatid adhesion at the apical ES in vivo. | Western Blotting | Rat | 23880313
 |
Intercellular adhesion molecule-2 is involved in apical ectoplasmic specialization dynamics during spermatogenesis in the rat.
Xiao, X; Cheng, CY; Mruk, DD
The Journal of endocrinology
216
73-86
2013
Show Abstract
In this study, we investigated the role of intercellular adhesion molecule-2 (ICAM2) in the testis. ICAM2 is a cell adhesion protein having important roles in cell migration, especially during inflammation when leukocytes cross the endothelium. Herein, we showed ICAM2 to be expressed by germ and Sertoli cells in the rat testis. When a monospecific antibody was used for immunolocalization experiments, ICAM2 was found to surround the heads of elongating/elongated spermatids in all stages of the seminiferous epithelial cycle. To determine whether ICAM2 is a constituent of apical ectoplasmic specialization (ES), co-immunoprecipitation and dual immunofluorescence staining were performed. Interestingly, ICAM2 was found to associate with β1-integrin, nectin-3, afadin, Src, proline-rich tyrosine kinase 2, annexin II, and actin. Following CdCl₂ treatment, ICAM2 was found to be upregulated during restructuring of the seminiferous epithelium, with round spermatids becoming increasingly immunoreactive for ICAM2 by 6-16 h. Interestingly, there was a loss in the binding of ICAM2 to actin during CdCl₂-induced germ cell loss, suggesting that a loss of ICAM2-actin interactions might have facilitated junction restructuring. Taken collectively, these results illustrate that ICAM2 plays an important role in apical ES dynamics during spermatogenesis. | | | 23097088
 |
c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution.
Xiao, X; Mruk, DD; Cheng, CY
American journal of physiology. Endocrinology and metabolism
304
E145-59
2013
Show Abstract
During spermatogenesis, extensive restructuring takes place at the cell-cell interface since developing germ cells migrate progressively from the basal to the adluminal compartment of the seminiferous epithelium. Since germ cells per se are not motile cells, their movement relies almost exclusively on the Sertoli cell. Nonetheless, extensive exchanges in signaling take place between these cells in the seminiferous epithelium. c-Yes, a nonreceptor protein tyrosine kinase belonging to the Src family kinases (SFKs) and a crucial signaling protein, was recently shown to be upregulated at the Sertoli cell-cell interface at the blood-testis barrier (BTB) at stages VIII-IX of the seminiferous epithelial cycle of spermatogenesis. It was also highly expressed at the Sertoli cell-spermatid interface known as apical ectoplasmic specialization (apical ES) at stage V to early stage VIII of the epithelial cycle during spermiogenesis. Herein, it was shown that the knockdown of c-Yes by RNAi in vitro and in vivo affected both Sertoli cell adhesion at the BTB and spermatid adhesion at the apical ES, causing a disruption of the Sertoli cell tight junction-permeability barrier function, germ cell loss from the seminiferous epithelium, and also a loss of spermatid polarity. These effects were shown to be mediated by changes in distribution and/or localization of adhesion proteins at the BTB (e.g., occludin, N-cadherin) and at the apical ES (e.g., nectin-3) and possibly the result of changes in the underlying actin filaments at the BTB and the apical ES. These findings implicate that c-Yes is a likely target of male contraceptive research. | Western Blotting | Rat | 23169788
 |
β1 integrin gene excision in the adult murine cardiac myocyte causes defective mechanical and signaling responses.
Li, R; Wu, Y; Manso, AM; Gu, Y; Liao, P; Israeli, S; Yajima, T; Nguyen, U; Huang, MS; Dalton, ND; Peterson, KL; Ross, RS
The American journal of pathology
180
952-62
2012
Show Abstract
How mechanical signals are transmitted in the cardiac myocyte is poorly understood. In this study, we produced a tamoxifen-inducible mouse model in which β1 integrin could be reduced specifically in the adult cardiomyocyte, so that the function of this integrin could be assessed in the postnatal and mechanically stressed heart. The expression of β1 integrin was reduced to 35% of control levels, but function remained normal at baseline. With aortic constriction, the knockout mice survived but had a blunted hypertrophic response. Integrin knockout myocytes, in contrast to controls, showed reduced integrin-linked kinase expression both at baseline and after hemodynamic stress; focal adhesion kinase expression was reduced after stress. Alterations in multiple signaling pathways were detected in the integrin knockout group after acute and chronic hemodynamic stress. Most remarkably, when we challenged the knockout mice with short-term loading, the robust responses of several kinases (extracellular signal-regulated kinase 1/2, p38, and Akt) evident in control mice were essentially abolished in the knockout mice. We also found that reduction of myocyte β1 integrin expression modified adrenergic-mediated signaling through extracellular signal-regulated kinase, p38, and Akt. Reduction of β1 integrin expression in the mature cardiac myocyte leads to a varied response compared with when this protein is reduced during either the embryonic or perinatal period. These results show that β1 integrin expression is required for proper mechanotransductive and adrenergic responses of the adult heart. | | | 22248583
 |
FAK promotes recruitment of talin to nascent adhesions to control cell motility.
Lawson, C; Lim, ST; Uryu, S; Chen, XL; Calderwood, DA; Schlaepfer, DD
The Journal of cell biology
196
223-32
2012
Show Abstract
Cell migration is a dynamic process that involves the continuous formation, maturation, and turnover of matrix-cell adhesion sites. New (nascent) adhesions form at the protruding cell edge in a tension-independent manner and are comprised of integrin receptors, signaling, and cytoskeletal-associated proteins. Integrins recruit focal adhesion kinase (FAK) and the cytoskeletal protein talin to nascent adhesions. Canonical models support a role for talin in mediating FAK localization and activation at adhesions. Here, alternatively, we show that FAK promotes talin recruitment to nascent adhesions occurring independently of talin binding to β1 integrins. The direct binding site for talin on FAK was identified, and a point mutation in FAK (E1015A) prevented talin association and talin localization to nascent adhesions but did not alter integrin-mediated FAK recruitment and activation at adhesions. Moreover, FAK E1015A inhibited cell motility and proteolytic talin cleavage needed for efficient adhesion dynamics. These results support an alternative linkage for FAK-talin interactions within nascent adhesions essential for the control of cell migration. | | | 22270917
 |