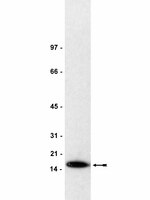
CSB-PGBD3 Mutations Cause Premature Ovarian Failure.
Qin, Y; Guo, T; Li, G; Tang, TS; Zhao, S; Jiao, X; Gong, J; Gao, F; Guo, C; Simpson, JL; Chen, ZJ
PLoS genetics
11
e1005419
2015
Show Abstract
Premature ovarian failure (POF) is a rare, heterogeneous disorder characterized by cessation of menstruation occurring before the age of 40 years. Genetic etiology is responsible for perhaps 25% of cases, but most cases are sporadic and unexplained. In this study, through whole exome sequencing in a non-consanguineous family having four affected members with POF and Sanger sequencing in 432 sporadic cases, we identified three novel mutations in the fusion gene CSB-PGBD3. Subsequently functional studies suggest that mutated CSB-PGBD3 fusion protein was impaired in response to DNA damage, as indicated by delayed or absent recruitment to damaged sites. Our data provide the first evidence that mutations in the CSB-PGBD3 fusion protein can cause human disease, even in the presence of functional CSB, thus potentially explaining conservation of the fusion protein for 43 My since marmoset. The localization of the CSB-PGBD3 fusion protein to UVA-induced nuclear DNA repair foci further suggests that the CSB-PGBD3 fusion protein, like many other proteins that can cause POF, modulates or participates in DNA repair. | | | 26218421
 |
A methyltransferase required for proper timing of the vernalization response in Arabidopsis.
Lee, J; Yun, JY; Zhao, W; Shen, WH; Amasino, RM
Proceedings of the National Academy of Sciences of the United States of America
112
2269-74
2015
Show Abstract
Prolonged exposure to winter cold enables flowering in many plant species through a process called vernalization. In Arabidopsis, vernalization results from the epigenetic silencing of the floral repressor flowering locus C (FLC) via a Polycomb Repressive Complex 2 (PRC2)-mediated increase in the density of the epigenetic silencing mark H3K27me3 at FLC chromatin. During cold exposure, a gene encoding a unique, cold-specific PRC2 component, vernalization insensitive 3 (VIN3), which is necessary for PRC2-mediated silencing of FLC, is induced. Here we show that set domain group 7 (SDG7) is required for proper timing of VIN3 induction and of the vernalization process. Loss of SDG7 results in a vernalization-hypersensitive phenotype, as well as more rapid cold-mediated up-regulation of VIN3. In the absence of cold, loss of SDG7 results in elevated levels of long noncoding RNAs, which are thought to participate in epigenetic repression of FLC. Furthermore, loss of SDG7 results in increased H3K27me3 deposition on FLC chromatin in the absence of cold exposure and enhanced H3K27me3 spreading during cold treatment. Thus, SDG7 is a negative regulator of vernalization, and loss of SDG7 creates a partially vernalized state without cold exposure. | | | 25605879
 |
Identification of histone H3 clipping activity in human embryonic stem cells.
Vossaert, L; Meert, P; Scheerlinck, E; Glibert, P; Van Roy, N; Heindryckx, B; De Sutter, P; Dhaenens, M; Deforce, D
Stem cell research
13
123-34
2014
Show Abstract
Posttranslational histone modifications are essential features in epigenetic regulatory networks. One of these modifications has remained largely understudied: regulated histone proteolysis. In analogy to the histone H3 clipping during early mouse embryonic stem cell differentiation, we report for the first time that also in human embryonic stem cells this phenomenon takes place in the two different analyzed cell lines. Employing complementary techniques, different cleavage sites could be identified, namely A21, R26 and residue 31. The enzyme responsible for this cleavage is found to be a serine protease. The formation of cleaved H3 follows a considerably variable pattern, depending on the timeframe, culture conditions and culture media applied. Contrary to earlier findings on H3 clipping, our results disconnect the link between declining Oct4 expression and H3 cleavage. | Western Blotting | Human | 24874291
 |
Antagonistic actions of Rcor proteins regulate LSD1 activity and cellular differentiation.
Upadhyay, G; Chowdhury, AH; Vaidyanathan, B; Kim, D; Saleque, S
Proceedings of the National Academy of Sciences of the United States of America
111
8071-6
2014
Show Abstract
Lysine-specific demethylase 1 (LSD1) demethylates nucleosomal histone H3 lysine 4 (H3K4) residues in collaboration with the corepressor CoREST/REST corepressor 1 (Rcor1) and regulates cell fates by epigenetically repressing gene targets. The balanced regulation of this demethylase, if any, is however unknown. We now demonstrate the actions of two other Rcor paralogs, Rcor2 and Rcor3, in regulating LSD1 enzymatic activity and biological function in hematopoietic cells. All three Rcor proteins interact with LSD1 and with the erythro-megakaryocytic transcription factor growth factor independence (Gfi)1b; however, whereas Rcor2, like Rcor1, facilitates LSD1-mediated nucleosomal demethylation, Rcor3 competitively inhibits this process. Appending the SANT2 domain of Rcor1 to Rcor3 confers the ability to facilitate LSD1-mediated demethylation on the chimeric Rcor protein. Consistent with their biochemical activities, endogenous Rcor1, Rcor2, and LSD1 promote differentiation, whereas Rcor3 opposes these processes. Recruitment of Rcor3 to cognate gene targets by Gfi1b and LSD1 leads to inhibition of H3K4 demethylation of chromatin and transcriptional derepression of these loci. Remarkably, profound alterations in Rcor1/3 levels during erythroid versus megakaryocytic differentiation potentiate antagonistic outcomes. In mature erythroid cells, a strong upsurge in Rcor3 and a sharp decline in Rcor1 levels counteract LSD1/Rcor1/2-mediated differentiation. In contrast, the opposite changes in Rcor1/3 levels in megakaryocytes favor differentiation and likely maintain homeostasis between these lineages. Overall, our results identify Rcor3 as a natural inhibitor of LSD1 and highlight a dual mechanism of regulating the enzymatic activity and restraining the epigenetic impact of this robust demethylase during hematopoietic differentiation. | Western Blotting | | 24843136
 |
Loss of Drosophila Ataxin-7, a SAGA subunit, reduces H2B ubiquitination and leads to neural and retinal degeneration.
Mohan, RD; Dialynas, G; Weake, VM; Liu, J; Martin-Brown, S; Florens, L; Washburn, MP; Workman, JL; Abmayr, SM
Genes & development
28
259-72
2014
Show Abstract
The Spt-Ada-Gcn5-acetyltransferase (SAGA) chromatin-modifying complex possesses acetyltransferase and deubiquitinase activities. Within this modular complex, Ataxin-7 anchors the deubiquitinase activity to the larger complex. Here we identified and characterized Drosophila Ataxin-7 and found that reduction of Ataxin-7 protein results in loss of components from the SAGA complex. In contrast to yeast, where loss of Ataxin-7 inactivates the deubiquitinase and results in increased H2B ubiquitination, loss of Ataxin-7 results in decreased H2B ubiquitination and H3K9 acetylation without affecting other histone marks. Interestingly, the effect on ubiquitination was conserved in human cells, suggesting a novel mechanism regulating histone deubiquitination in higher organisms. Consistent with this mechanism in vivo, we found that a recombinant deubiquitinase module is active in the absence of Ataxin-7 in vitro. When we examined the consequences of reduced Ataxin-7 in vivo, we found that flies exhibited pronounced neural and retinal degeneration, impaired movement, and early lethality. | | | 24493646
 |
Molecular targets of chromatin repressive mark H3K9me3 in primate progenitor cells within adult neurogenic niches.
Foret, MR; Sandstrom, RS; Rhodes, CT; Wang, Y; Berger, MS; Lin, CH
Frontiers in genetics
5
252
2014
Show Abstract
Histone 3 Lysine 9 (H3K9) methylation is known to be associated with pericentric heterochromatin and important in genomic stability. In this study, we show that trimethylation at H3K9 (H3K9me3) is enriched in an adult neural stem cell niche- the subventricular zone (SVZ) on the walls of the lateral ventricle in both rodent and non-human primate baboon brain. Previous studies have shown that there is significant correlation between baboon and human regarding genomic similarity and brain structure, suggesting that findings in baboon are relevant to human. To understand the function of H3K9me3 in this adult neurogenic niche, we performed genome-wide analyses using ChIP-Seq (chromatin immunoprecipitation and deep-sequencing) and RNA-Seq for in vivo SVZ cells purified from baboon brain. Through integrated analyses of ChIP-Seq and RNA-Seq, we found that H3K9me3-enriched genes associated with cellular maintenance, post-transcriptional and translational modifications, signaling pathways, and DNA replication are expressed, while genes involved in axon/neuron, hepatic stellate cell, or immune-response activation are not expressed. As neurogenesis progresses in the adult SVZ, cell fate restriction is essential to direct proper lineage commitment. Our findings highlight that H3K9me3 repression in undifferentiated SVZ cells is engaged in the maintenance of cell type integrity, implicating a role for H3K9me3 as an epigenetic mechanism to control cell fate transition within this adult germinal niche. | Fluorescence Activated Cell Sorting (FACS) | | 25126093
 |
Epigenetic regulation by chromatin activation mark H3K4me3 in primate progenitor cells within adult neurogenic niche.
Sandstrom, RS; Foret, MR; Grow, DA; Haugen, E; Rhodes, CT; Cardona, AE; Phelix, CF; Wang, Y; Berger, MS; Lin, CH
Scientific reports
4
5371
2014
Show Abstract
Histone 3 lysine 4 trimethylation (H3K4me3) is known to be associated with transcriptionally active or poised genes and required for postnatal neurogenesis within the subventricular zone (SVZ) in the rodent model. Previous comparisons have shown significant correlation between baboon (Papio anubis) and human brain. In this study, we demonstrate that chromatin activation mark H3K4me3 is present in undifferentiated progenitor cells within the SVZ of adult baboon brain. To identify the targets and regulatory role of H3K4me3 within the baboon SVZ, we developed a technique to purify undifferentiated SVZ cells while preserving the endogenous nature without introducing culture artifact to maintain the in vivo chromatin state for genome-wide studies (ChIP-Seq and RNA-Seq). Overall, H3K4me3 is significantly enriched for genes involved in cell cycle, metabolism, protein synthesis, signaling pathways, and cancer mechanisms. Additionally, we found elevated levels of H3K4me3 in the MRI-classified SVZ-associated Glioblastoma Multiforme (GBM), which has a transcriptional profile that reflects the H3K4me3 modifications in the undifferentiated progenitor cells of the baboon SVZ. Our findings highlight the importance of H3K4me3 in coordinating distinct networks and pathways for life-long neurogenesis, and suggest that subtypes of GBM could occur, at least in part, due to aberrant H3K4me3 epigenetic regulation. | | | 24947819
 |
Influence of Egr-1 in cardiac tissue-derived mesenchymal stem cells in response to glucose variations.
Bastianelli, D; Siciliano, C; Puca, R; Coccia, A; Murdoch, C; Bordin, A; Mangino, G; Pompilio, G; Calogero, A; De Falco, E
BioMed research international
2014
254793
2014
Show Abstract
Mesenchymal stem cells (MSCs) represent a promising cell population for cell therapy and regenerative medicine applications. However, how variations in glucose are perceived by MSC pool is still unclear. Since, glucose metabolism is cell type and tissue dependent, this must be considered when MSCs are derived from alternative sources such as the heart. The zinc finger transcription factor Egr-1 is an important early response gene, likely to play a key role in the glucose-induced response. Our aim was to investigate how short-term changes in in vitro glucose concentrations affect multipotent cardiac tissue-derived MSCs (cMSCs) in a mouse model of Egr-1 KO (Egr-1(-/-)). Results showed that loss of Egr-1 does not significantly influence cMSC proliferation. In contrast, responses to glucose variations were observed in wt but not in Egr-1(-/-) cMSCs by clonogenic assay. Phenotype analysis by RT-PCR showed that cMSCs Egr-1(-/-) lost the ability to regulate the glucose transporters GLUT-1 and GLUT-4 and, as expected, the Egr-1 target genes VEGF, TGF β -1, and p300. Acetylated protein levels of H3 histone were impaired in Egr-1(-/-) compared to wt cMSCs. We propose that Egr-1 acts as immediate glucose biological sensor in cMSCs after a short period of stimuli, likely inducing epigenetic modifications. | Western Blotting | | 24967343
 |
Down-Regulation of miR-129-5p and the let-7 Family in Neuroendocrine Tumors and Metastases Leads to Up-Regulation of Their Targets Egr1, G3bp1, Hmga2 and Bach1.
Døssing, KB; Binderup, T; Kaczkowski, B; Jacobsen, A; Rossing, M; Winther, O; Federspiel, B; Knigge, U; Kjær, A; Friis-Hansen, L
Genes
6
1-21
2014
Show Abstract
Expression of miRNAs in Neuroendocrine Neoplasms (NEN) is poorly characterized. We therefore wanted to examine the miRNA expression in Neuroendocrine Tumors (NETs), and identify their targets and importance in NET carcinogenesis. miRNA expression in six NEN primary tumors, six NEN metastases and four normal intestinal tissues was characterized using miRNA arrays, and validated by in-situ hybridization and qPCR. Among the down-regulated miRNAs miR-129-5p and the let-7f/let-7 family, were selected for further characterization. Transfection of miR-129-5p inhibited growth of a pulmonary and an intestinal carcinoid cell line. Analysis of mRNA expression changes identified EGR1 and G3BP1 as miR-129-5p targets. They were validated by luciferase assay and western blotting, and found robustly expressed in NETs by immunohistochemistry. Knockdown of EGR1 and G3BP1 mimicked the growth inhibition induced by miR-129-5p. let-7 overexpression inhibited growth of carcinoid cell lines, and let-7 inhibition increased protein content of the transcription factor BACH1 and its targets MMP1 and HMGA2, all known to promote bone metastases. Immunohistochemistry analysis revealed that let-7 targets are highly expressed in NETs and metastases. We found down-regulation of miR-129-5p and the let-7 family, and identified new neuroendocrine specific targets for these miRNAs, which contributes to the growth and metastatic potential of these tumors. | | | 25546138
 |
Enhanced trimethylation of histone h3 mediates impaired expression of hepatic glucose 6-phosphatase expression in offspring from rat dams exposed to hypoxia during pregnancy.
Osumek, JE; Revesz, A; Morton, JS; Davidge, ST; Hardy, DB
Reproductive sciences (Thousand Oaks, Calif.)
21
112-21
2014
Show Abstract
Given that hepatic glucose 6-phosphatase (G6Pase, involved in gluconeogenesis) has been demonstrated to be altered long term in animal models of intrauterine growth restriction (IUGR), we hypothesized that hypoxia in utero may regulate G6Pase expression via epigenetic mechanisms. To address this further, a rat model of maternal hypoxia leading to IUGR and impaired liver growth was utilized. In the 12-month-old male offspring of pregnant rat dams exposed to 11.5% atmospheric oxygen from gestational day (gd) 15 to gd 21, nonfasting glucose was lower in association with decreased hepatic G6Pase messenger RNA and protein levels. This was concomitant with enhanced methylation of histone H3 [K9] surrounding the promoter of G6Pase. Moreover, when McA-RH7777 hepatoma cells were exposed to various concentrations of oxygen for 48 hours, we observed an oxygen-dependent decrease in G6Pase expression associated with enhanced histone H3 [K9] methylation. Collectively, these results indicate that hypoxia directly and indirectly impairs G6Pase expression through enhanced methylation of histone H3 [K9]. | Western Blotting | | 23744881
 |