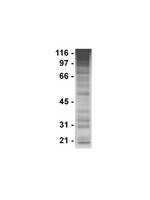
Sorafenib induces cathepsin B-mediated apoptosis of bladder cancer cells by regulating the Akt/PTEN pathway. The Akt inhibitor, perifosine, enhances the sorafenib-induced cytotoxicity against bladder cancer cells.
Amantini, C; Morelli, MB; Santoni, M; Soriani, A; Cardinali, C; Farfariello, V; Eleuteri, AM; Bonfili, L; Mozzicafreddo, M; Nabissi, M; Cascinu, S; Santoni, G
Oncoscience
2
395-409
2015
Show Abstract
Sorafenib, a tyrosine kinase inhibitor, has been demonstrated to exert anti-tumor effects. However, the molecular mechanisms underlying its effects on bladder cancer remain unknown. Here, we evaluated the mechanisms responsible for the sorafenib-induced anti-tumor effects on 5637 and T24 bladder cancer cells. We demonstrated that sorafenib reduces cell viability, stimulates lysosome permeabilization and induces apoptosis of bladder cancer cells. These effects are dependent by the activation of cathepsin B released from lysosomes. The sorafenib-increased cathepsin B activity induced the proteolysis of Bid into tBid that stimulates the intrinsic pathway of apoptosis characterized by mitochondrial membrane depolarization, oxygen radical generation and cytochrome c release. Moreover, we found that cathepsin B enzymatic activity, induced by sorafenib, is dependent on its dephosphorylation via PTEN activation and Akt inactivation. Pretreatment with orthovanadate rescued bladder cancer cells from apoptosis. In addition, the Akt inhibitor perifosine increased the sensitivity of bladder cancer cells to sorafenib-induced cytotoxicity. Overall, our results show that apoptotic cell death induced by sorafenib in bladder cancer cells is dependent on cathepsin B activity and involved PTEN and Akt signaling pathways. The Akt inhibitor perifosine increased the cytotoxic effects of sorafenib in bladder cancer cells. | 26097873
 |
Rho-ROCK signaling differentially regulates chondrocyte spreading on fibronectin and bone sialoprotein.
Gill, KS; Beier, F; Goldberg, HA
American journal of physiology. Cell physiology
295
C38-49
2008
Show Abstract
The mammalian growth plate is a dynamic structure rich in extracellular matrix (ECM). Interactions of growth plate chondrocytes with ECM proteins regulate cell behavior. In this study, we compared chondrocyte adhesion and spreading dynamics on fibronectin (FN) and bone sialoprotein (BSP). Chondrocyte adhesion and spreading were also compared with fibroblasts to analyze potential cell-type-specific effects. Chondrocyte adhesion to BSP is independent of posttranslational modifications but is dependent on the RGD sequence in BSP. Whereas chondrocytes and fibroblasts adhered at similar levels on FN and BSP, cells displayed more actin-dependent spread on FN despite a 16x molar excess of BSP adsorbed to plastic. To identify intracellular mediators responsible for this difference in spreading, we investigated focal adhesion kinase (FAK)-Src and Rho-Rho kinase (ROCK) signaling. Although activated FAK localized to the vertices of adhered chondrocytes, levels of FAK activation did not correlate with the extent of spreading. Furthermore, Src inhibition reduced chondrocyte spreading on both FN and BSP, suggesting that FAK-Src signaling is not responsible for less cell spreading on BSP. In contrast, inhibition of Rho and ROCK in chondrocytes increased cell spreading on BSP and membrane protrusiveness on FN but did not affect cell adhesion. In fibroblasts, Rho inhibition increased fibroblast spreading on BSP while ROCK inhibition changed membrane protrusiveness of FN and BSP. In summary, we identify a novel role for Rho-ROCK signaling in regulating chondrocyte spreading and demonstrate both cell- and matrix molecule-specific mechanisms controlling cell spreading. Full Text Article | 18463228
 |
Protein tyrosine phosphatases and signalling.
Stoker, Andrew W
J. Endocrinol., 185: 19-33 (2005)
2005
Show Abstract
A cornerstone of many cell-signalling events rests on reversible phosphorylation of tyrosine residues on proteins. The reversibility relies on the coordinated actions of protein tyrosine kinases and protein tyrosine phosphatases (PTPs), both of which exist as large protein families. This review focuses on the rapidly evolving field of the PTPs. We now know that rather than simply scavenging phosphotyrosine, the PTPs specifically regulate a wide range of signalling pathways. To illustrate this and to highlight current areas of agreement and contention in the field, this review will present our understanding of PTP action in selected areas and will present current knowledge surrounding the regulatory mechanisms that control PTP enzymes themselves. It will be seen that PTPs control diverse processes such as focal adhesion dynamics, cell-cell adhesion and insulin signalling, and their own actions are in turn regulated by dimerisation, phosphorylation and reversible oxidation. | 15817824
 |
Anchorage-dependent cell growth: tyrosine kinases and phosphatases meet redox regulation.
Chiarugi, Paola and Giannoni, Elisa
Antioxid. Redox Signal., 7: 578-92 (2005)
2005
Show Abstract
Recent data have provided new insight concerning the regulation of nontransformed cell proliferation in response to both soluble growth factors and adhesive cues. Nontransformed cells are anchorage-dependent for the execution of the complete mitotic program and cannot avoid the concomitant signals starting from mitogenic molecules, as growth factors, and adhesive agents belonging to the extracellular matrix. Protein tyrosine kinases (PTKs) and phosphotyrosine phosphatases (PTPs) together with soluble small molecules have been included among intracellular signal transducers of growth factor and extracellular matrix receptors. Reactive oxygen species retain a key role during both growth factor and integrin receptor signaling, and these second messengers are recognized to be a synergistic point of confluence for anchorage-dependent growth signaling. Redox-regulated proteins include PTPs and PTKs, although with opposite regulation of enzymatic activity. Transient oxidation of PTPs leads to their inactivation, through the formation of an intramolecular S-S bridge. Conversely, oxidation of PTKs leads to their activation, either by direct SH modification or, indirectly, by concomitant inhibition of PTPs that leads to sustained activation of PTKs. This review will focus on the redox regulation of PTPs and PTKs during anchorage-dependent cell growth and its implications for tumor biology. | 15890002
 |
Monoclonal antibodies to phosphotyrosine.
Glenney, J R, et al.
J. Immunol. Methods, 109: 277-85 (1988)
1988
Show Abstract
Phosphotyrosine coupled to KLH, BSA, and OVA was used for the production and screening of antibodies to phosphotyrosine. 800 hybridomas secreting antibodies that bound to phosphotyrosine were detected by ELISA. The most reactive 100 of these 800 were tested subsequently for their ability to bind phosphotyrosine-containing proteins on Western blots. Eight stable hybridoma cell lines were selected for further study, cloned by limiting dilution, and grown as ascites. These antibodies were purified by three different methods, and it was found that affinity chromatography on phosphotyrosine-affigel provided the most rapid and effective method. Many phosphotyrosine-containing proteins were detected by using these antibodies in Western blotting and immunoaffinity purification procedures. Binding of anti-phosphotyrosine antibody could be competed by phosphotyrosine or phenylphosphate but not by phosphoserine, phosphothreonine, or free phosphate. These antibodies should be useful for the identification and purification of proteins phosphorylated on tyrosine residues in transformed and growth factor-treated cells. | 2452204
 |