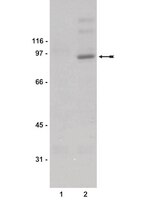
High STAT1 mRNA levels but not its tyrosine phosphorylation are associated with macrophage infiltration and bad prognosis in breast cancer.
Tymoszuk, P; Charoentong, P; Hackl, H; Spilka, R; Müller-Holzner, E; Trajanoski, Z; Obrist, P; Revillion, F; Peyrat, JP; Fiegl, H; Doppler, W
BMC cancer
14
257
2014
Show Abstract
STAT1 has been attributed a function as tumor suppressor. However, in breast cancer data from microarray analysis indicated a predictive value of high mRNA expression levels of STAT1 and STAT1 target genes belonging to the interferon-related signature for a poor response to therapy. To clarify this issue we have determined STAT1 expression levels and activation by different methods, and investigated their association with tumor infiltration by immune cells. Additionally, we evaluated the interrelationship of these parameters and their significance for predicting disease outcome.Expression of STAT1, its target genes SOCS1, IRF1, CXCL9, CXCL10, CXCL11, IFIT1, IFITM1, MX1 and genes characteristic for immune cell infiltration (CD68, CD163, PD-L1, PD-L2, PD-1, CD45, IFN-γ, FOXP3) was determined by RT-PCR in two independent cohorts comprising 132 breast cancer patients. For a subset of patients, protein levels of total as well as serine and tyrosine-phosphorylated STAT1 were ascertained by immunohistochemistry or immunoblotting and protein levels of CXCL10 by ELISA.mRNA expression levels of STAT1 and STAT1 target genes, as well as protein levels of total and serine-phosphorylated STAT1 correlated with each other in neoplastic tissue. However, there was no association between tumor levels of STAT1 mRNA and tyrosine-phosphorylated STAT1 and between CXCL10 serum levels and CXCL10 expression in the tumor. Tumors with increased STAT1 mRNA amounts exhibited elevated expression of genes characteristic for tumor-associated macrophages and immunosuppressive T lymphocytes. Survival analysis revealed an association of high STAT1 mRNA levels and bad prognosis in both cohorts. A similar prognostically relevant correlation with unfavorable outcome was evident for CXCL10, MX1, CD68, CD163, IFN-γ, and PD-L2 expression in at least one collective. By contrast, activation of STAT1 as assessed by the level of STAT1-Y701 phosphorylation was linked to positive outcome. In multivariate Cox regression, the predictive power of STAT1 mRNA expression was lost when including expression of CXCL10, MX1 and CD68 as confounders.Our study confirms distinct prognostic relevance of STAT1 expression levels and STAT1 tyrosine phosphorylation in breast cancer patients and identifies an association of high STAT1 levels with elevated expression of STAT1 target genes and markers for infiltrating immune cells. | Western Blotting | | 24725474
 |
Dual modulation of type I interferon response by bluetongue virus.
Doceul, V; Chauveau, E; Lara, E; Bréard, E; Sailleau, C; Zientara, S; Vitour, D
Journal of virology
88
10792-802
2014
Show Abstract
Bluetongue virus (BTV) is a double-stranded RNA (dsRNA) virus that causes an economically important disease in ruminants. BTV infection is a strong inducer of type I interferon (IFN-I) in multiple cell types. It has been shown recently that BTV and, more specifically, the nonstructural protein NS3 of BTV are able to modulate the IFN-I synthesis pathway. However, nothing is known about the ability of BTV to counteract IFN-I signaling. Here, we investigated the effect of BTV on the IFN-I response pathway and, more particularly, the Janus tyrosine kinase (JAK)/signal transducer and activator of transcription protein (STAT) signaling pathway. We found that BTV infection triggered the expression of IFN-stimulated genes (ISGs) in A549 cells. However, when BTV-infected cells were stimulated with external IFN-I, we showed that activation of the IFN-stimulated response element (ISRE) promoter and expression of ISGs were inhibited. We found that this inhibition involved two different mechanisms that were dependent on the time of infection. After overnight infection, BTV blocked specifically the phosphorylation and nuclear translocation of STAT1. This inhibition correlated with the redistribution of STAT1 in regions adjacent to the nucleus. At a later time point of infection, BTV was found to interfere with the activation of other key components of the JAK/STAT pathway and to induce the downregulation of JAK1 and TYK2 protein expression. Overall, our study indicates for the first time that BTV is able to interfere with the JAK/STAT pathway to modulate the IFN-I response.Bluetongue virus (BTV) causes a severe disease in ruminants and has an important impact on the livestock economy in areas of endemicity such as Africa. The emergence of strains, such as serotype 8 in Europe in 2006, can lead to important economic losses due to commercial restrictions and prophylactic measures. It has been known for many years that BTV is a strong inducer of type I interferon (IFN-I) in vitro and in vivo in multiple cell types. However, the ability of BTV to interact with the IFN-I system remains unclear. Here, we report that BTV is able to modulate the IFN-I response by interfering with the Janus tyrosine kinase (JAK)/signal transducer and activator of transcription protein (STAT) signaling pathway. These findings contribute to knowledge of how BTV infection interferes with the host's innate immune response and becomes pathogenic. This will also be important for the design of efficacious vaccine candidates. | | | 25008919
 |
STAT2 deficiency and susceptibility to viral illness in humans.
Hambleton, S; Goodbourn, S; Young, DF; Dickinson, P; Mohamad, SM; Valappil, M; McGovern, N; Cant, AJ; Hackett, SJ; Ghazal, P; Morgan, NV; Randall, RE
Proceedings of the National Academy of Sciences of the United States of America
110
3053-8
2013
Show Abstract
Severe infectious disease in children may be a manifestation of primary immunodeficiency. These genetic disorders represent important experiments of nature with the capacity to elucidate nonredundant mechanisms of human immunity. We hypothesized that a primary defect of innate antiviral immunity was responsible for unusually severe viral illness in two siblings; the proband developed disseminated vaccine strain measles following routine immunization, whereas an infant brother died after a 2-d febrile illness from an unknown viral infection. Patient fibroblasts were indeed abnormally permissive for viral replication in vitro, associated with profound failure of type I IFN signaling and absence of STAT2 protein. Sequencing of genomic DNA and RNA revealed a homozygous mutation in intron 4 of STAT2 that prevented correct splicing in patient cells. Subsequently, other family members were identified with the same genetic lesion. Despite documented infection by known viral pathogens, some of which have been more severe than normal, surviving STAT2-deficient individuals have remained generally healthy, with no obvious defects in their adaptive immunity or developmental abnormalities. These findings imply that type I IFN signaling [through interferon-stimulated gene factor 3 (ISGF3)] is surprisingly not essential for host defense against the majority of common childhood viral infections. | | | 23391734
 |
Porcine reproductive and respiratory syndrome virus Nsp1β inhibits interferon-activated JAK/STAT signal transduction by inducing karyopherin-α1 degradation.
Wang, Rong, et al.
J. Virol., 87: 5219-28 (2013)
2013
Show Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) inhibits the interferon-mediated antiviral response. Type I interferons (IFNs) induce the expression of IFN-stimulated genes by activating phosphorylation of both signal transducer and activator of transcription 1 (STAT1) and STAT2, which form heterotrimers (interferon-stimulated gene factor 3 [ISGF3]) with interferon regulatory factor 9 (IRF9) and translocate to the nucleus. PRRSV Nsp1β blocks the nuclear translocation of the ISGF3 complex by an unknown mechanism. In this study, we discovered that Nsp1β induced the degradation of karyopherin-α1 (KPNA1, also called importin-α5), which is known to mediate the nuclear import of ISGF3. Overexpression of Nsp1β resulted in a reduction of KPNA1 levels in a dose-dependent manner, and treatment of the cells with the proteasome inhibitor MG132 restored KPNA1 levels. Furthermore, the presence of Nsp1β induced an elevation of KPNA1 ubiquitination and a shortening of its half-life. Our analysis of Nsp1β deletion constructs showed that the N-terminal domain of Nsp1β was involved in the ubiquitin-proteasomal degradation of KPNA1. A nucleotide substitution resulting in an amino acid change from valine to isoleucine at residue 19 of Nsp1β diminished its ability to induce KPNA1 degradation and to inhibit IFN-mediated signaling. Interestingly, infection of MARC-145 cells by PRRSV strains VR-2332 and VR-2385 also resulted in KPNA1 reduction, whereas infection by an avirulent strain, Ingelvac PRRS modified live virus (MLV), did not. MLV Nsp1β had no effect on KPNA1; however, a mutant with an amino acid change at residue 19 from isoleucine to valine induced KPNA1 degradation. These results indicate that Nsp1β blocks ISGF3 nuclear translocation by inducing KPNA1 degradation and that valine-19 in Nsp1β correlates with the inhibition. | | | 23449802
 |
Induction of type I interferons by a novel porcine reproductive and respiratory syndrome virus isolate.
Nan, Yuchen, et al.
Virology, 432: 261-70 (2012)
2012
Show Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is known to interfere with the signaling of type I interferons (IFNs). Here we found PRRSV A2MC2 induced type I IFNs in cultured cells. A2MC2 replication in MARC-145 cells resulted in the synthesis of IFN-α2 protein, transcript elevation of the IFN-stimulated genes ISG15 and ISG56, and the proteins of the signal transducer and activator of transcription 2 (STAT2) and ISG56. A2MC2 infection of primary porcine pulmonary alveolar macrophages (PAMs) also led to the elevation of the two proteins, but had little cytopathic effect. Furthermore, A2MC2 infection of MARC-145 or PAM cells had no detectable inhibitory effect on the ability of IFN-α to induce an antiviral response. Sequencing analysis indicated that A2MC2 was closely related to VR-2332 and Ingelvac PRRS MLV with an identity of 99.8% at the nucleotide level. The identification of this IFN-inducing PRRSV isolate may be beneficial for vaccine development against PRRS. | | | 22704065
 |
The anti-interferon activity of conserved viral dUTPase ORF54 is essential for an effective MHV-68 infection.
Leang, RS; Wu, TT; Hwang, S; Liang, LT; Tong, L; Truong, JT; Sun, R
PLoS pathogens
7
e1002292
2011
Show Abstract
Gammaherpesviruses such as KSHV and EBV establish lifelong persistent infections through latency in lymphocytes. These viruses have evolved several strategies to counteract the various components of the innate and adaptive immune systems. We conducted an unbiased screen using the genetically and biologically related virus, MHV-68, to find viral ORFs involved in the inhibition of type I interferon signaling and identified a conserved viral dUTPase, ORF54. Here we define the contribution of ORF54 in type I interferon inhibition by ectopic expression and through the use of genetically modified MHV-68. ORF54 and an ORF54 lacking dUTPase enzymatic activity efficiently inhibit type I interferon signaling by inducing the degradation of the type I interferon receptor protein IFNAR1. Subsequently, we show in vitro that the lack of ORF54 causes a reduction in lytic replication in the presence of type I interferon signaling. Investigation of the physiological consequence of IFNAR1 degradation and importance of ORF54 during MHV-68 in vivo infection demonstrates that ORF54 has an even greater impact on persistent infection than on lytic replication. MHV-68 lacking ORF54 expression is unable to efficiently establish latent infection in lymphocytes, although it replicates relatively normally in lung tissues. However, infection of IFNAR-/- mice alleviates this phenotype, emphasizing the specific role of ORF54 in type I interferon inhibition. Infection of mice and cells by a recombinant MHV-68 virus harboring a site specific mutation in ORF54 rendering the dUTPase inactive demonstrates that dUTPase enzymatic activity is not required for anti-interferon function of ORF54. Moreover, we find that dUTPase activity is dispensable at all stages of MHV-68 infection analyzed. Overall, our data suggest that ORF54 has evolved anti-interferon activity in addition to its dUTPase enzymatic activity, and that it is actually the anti-interferon role that renders ORF54 critical for establishing an effective persistent infection of MHV-68. | Western Blotting | Mouse | 21998588
 |
Precision-cut slice cultures of tumors from MMTV-neu mice for the study of the ex vivo response to cytokines and cytotoxic drugs.
Nirmala Parajuli, Wolfgang Doppler
In vitro cellular developmental biology. Animal
45
442-50
2009
Show Abstract
Ex vivo analysis of signaling pathways operating in tumor tissue is complicated by the three-dimensional structure, in particular by stroma-epithelial interactions. Studies performed with pure populations of tumor cells usually do not take into account this issue. One possibility to preserve the tissue architecture is the use of tumor slices. However, diffusion of oxygen and nutrients may become limiting factors, resulting in decreased cell viability and change of tissue morphology, especially after long-term incubation of slices. By using precision cut slices of defined thickness, we were able to establish culture conditions for tumor material obtained from MMTV-neu transgenic mice, which allow the study of the action of cytokines and cytotoxic drugs for up to 24 h. A slice thickness of 160 mum was found to be optimal for viability and handling of material. These slices were highly responsive to the action of the cytokine IFN-gamma, as evident form the increase of pY701 STAT1, detected by both immunohistochemistry and western blotting, and by the increase of mRNA levels of the IFN-gamma response genes IRF-1, SOCS-1, and STAT1, analyzed by reverse transcriptase-polymerase chain reaction. Furthermore, induction of apoptosis and increase of DNA damage could be monitored after treatment with IFN-gamma or doxorubicin. The slices were also a convenient source for the establishment of explant cultures of tumor epithelial cells. It is concluded that cultivation of precision-cut tumor slices provides a convenient way for the ex vivo molecular analysis of MMTV-neu tumor tissue under conditions which closely simulate the situation in vivo and can provide an alternative to in vivo experiments. | | | 19533258
 |
Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation.
Hauri-Hohl, MM; Keller, MP; Gill, J; Hafen, K; Pachlatko, E; Boulay, T; Peter, A; Holländer, GA; Krenger, W
Blood
109
4080-8
2007
Show Abstract
Acute graft-versus-host disease (aGVHD) impairs thymus-dependent T-cell regeneration in recipients of allogeneic bone marrow transplants through yet to be defined mechanisms. Here, we demonstrate in mice that MHC-mismatched donor T cells home into the thymus of unconditioned recipients. There, activated donor T cells secrete IFN-gamma, which in turn stimulates the programmed cell death of thymic epithelial cells (TECs). Because TECs themselves are competent and sufficient to prime naive allospecific T cells and to elicit their effector function, the elimination of host-type professional antigen-presenting cells (APCs) does not prevent donor T-cell activation and TEC apoptosis, thus precluding normal thymopoiesis in transplant recipients. Hence, strategies that protect TECs may be necessary to improve immune reconstitution following allogeneic bone marrow transplantation. Full Text Article | | | 17213290
 |
Basal expression levels of IFNAR and Jak-STAT components are determinants of cell-type-specific differences in cardiac antiviral responses.
Zurney, J; Howard, KE; Sherry, B
Journal of virology
81
13668-80
2007
Show Abstract
Viral myocarditis is an important human disease, and reovirus-induced murine myocarditis provides an excellent model system for study. Cardiac myocytes, like neurons in the central nervous system, are not replenished, yet there is no cardiac protective equivalent to the blood-brain barrier. Thus, cardiac myocytes may have evolved a unique antiviral response relative to readily replenished cell types, such as cardiac fibroblasts. Our previous comparisons of these two cell types revealed a conundrum: reovirus T3D induces more beta-interferon (IFN-beta) mRNA in cardiac myocytes, yet there is a greater induction of IFN-stimulated genes (ISGs) in cardiac fibroblasts. Here, we investigated possible underlying molecular determinants. We found that greater basal expression of IFN-beta in cardiac myocytes results in greater basal activated nuclear STAT1 and STAT2 and greater basal ISG mRNA expression and provides greater basal antiviral protection relative to cardiac fibroblasts. Conversely, cardiac fibroblasts express greater basal IFN-alpha/beta receptor 1 (IFNAR1) and greater basal cytoplasmic Jak1, Tyk2, STAT2, and IRF9, leading to a greater increase in reovirus T3D- or IFN-induced nuclear activated STAT1 and STAT2 and greater induction of ISGs for a greater IFN-induced antiviral protection relative to cardiac myocytes. Our results suggest that high basal IFN-beta expression in cardiac myocytes prearms this vulnerable, nonreplenishable cell type, while high basal expression of IFNAR1 and latent Jak-STAT components in adjacent cardiac fibroblasts renders these cells more responsive to IFN and prevents them from inadvertently serving as a reservoir for viral replication and spread to cardiac myocytes. These studies provide the first indication of an integrated network of cell-type-specific innate immune components for organ protection. Full Text Article | | | 17942530
 |
Mapuera virus, a rubulavirus that inhibits interferon signalling in a wide variety of mammalian cells without degrading STATs.
Hagmaier, K; Stock, N; Precious, B; Childs, K; Wang, LF; Goodbourn, S; Randall, RE
The Journal of general virology
88
956-66
2007
Show Abstract
Mapuera virus (MPRV) is a paramyxovirus that was originally isolated from bats, but its host range remains unknown. It was classified as a member of the genus Rubulavirus on the basis of structural and genetic features. Like other rubulaviruses it encodes a V protein (MPRV/V) that functions as an interferon (IFN) antagonist. Here we show that MPRV/V differs from the IFN antagonists of other rubulaviruses in that it does not induce the proteasomal degradation of STAT proteins, key factors in the IFN signalling cascade. Rather, MPRV/V prevents the nuclear translocation of STATs in response to IFN stimulation and inhibits the formation of the transcription factor complex ISGF3. We also show that MPRV/V blocks IFN signalling in cells from diverse mammalian species and discuss the IFN response as a barrier to cross-species infections. | | | 17325370
 |