17-10188 Sigma-AldrichLentiBrite™ RFP-LC3 Control Mutant Lentiviral Biosensor
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| Key Applications | Detection Methods |
|---|---|
| TFX, IF, ICC | Fluorescent |
| Description | |
|---|---|
| Catalogue Number | 17-10188 |
| Trade Name |
|
| Description | LentiBrite™ RFP-LC3 Control Mutant Lentiviral Biosensor |
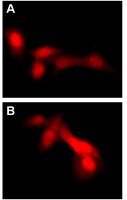
| Overview | Read our application note in Nature Methods! http://www.nature.com/app_notes/nmeth/2012/121007/pdf/an8620.pdf (Click Here!) Learn more about the advantages of our LentiBrite Lentiviral Biosensors! Click Here Biosensors can be used to detect the presence/absence of a particular protein as well as the subcellular location of that protein within the live state of a cell. Fluorescent tags are often desired as a means to visualize the protein of interest within a cell by either fluorescent microscopy or time-lapse video capture. Visualizing live cells without disruption allows researchers to observe cellular conditions in real time. Lentiviral vector systems are a popular research tool used to introduce gene products into cells. Lentiviral transfection has advantages over non-viral methods such as chemical-based transfection including higher-efficiency transfection of dividing and non-dividing cells, long-term stable expression of the transgene, and low immunogenicity. EMD Millipore is introducing LentiBrite™ Lentiviral Biosensors, a new suite of pre-packaged lentiviral particles encoding important and foundational proteins of autophagy, apoptosis, and cell structure for visualization under different cell/disease states in live cell and in vitro analysis. • Pre-packaged, fluorescently-tagged with GFP & RFP • Higher efficiency transfection as compared to traditional chemical-based and other non-viral-based transfection methods • Ability to transfect dividing, non-dividing, and difficult-to-transfect cell types, such as primary cells or stem cells •Non-disruptive towards cellular function EMD Millipore’s LentiBrite™ RFP-LC3-G120A lentiviral particles serve as a negative control alongside RFP-LC3 wild-type (catalog # 17-10143) for live cell analysis of autophagy. |
| Alternate Names |
|
| Background Information | Autophagy, a stress-induced degradative pathway, plays a role in many diseases, including cancer, neurodegeneration, and infections. Members of the LC3 family play a key role in the maturation of the autophagosome, the central organelle of autophagy. LC3 precursors are proteolytically processed to form cytosolic LC3-I. Upon initiation of autophagy, the C-terminal glycine is modified by addition of phosphatidylethanolamine (PE) to form LC3-II, which translocates to autophagosomes in a punctate distribution. DNA constructs encoding fluorescent proteins fused to LC3 are widely employed for monitoring autophagosome formation by fluorescence microscopy. A mutant form of LC3 with the C-terminal glycine changed to alanine (LC3-G120A) is unable to accept the PE modification and fails to translocate to the autophagosome upon induction of autophagy. EMD Millipore’s LentiBrite™ RFP-LC3-G120A lentiviral particles serve as a negative control alongside RFP-LC3 wild-type (catalog # 17-10143) for live cell analysis of autophagy. |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Detection method | Fluorescent |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Gene Symbol |
|
| Purification Method | PEG precipitation |
| UniProt Number | |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements | |
|---|---|
| Quality Assurance | Evaluated by transduction of HT-1080 cells and fluorescent imaging performed for assessment of transduction efficiency. |
| Usage Statement |
|
| Packaging Information | |
|---|---|
| Material Size | 1 vial (minimum of 3 x 10E8 IFU/mL) |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 17-10188 | 04053252549236 |
Documentation
LentiBrite™ RFP-LC3 Control Mutant Lentiviral Biosensor SDS
| Title |
|---|