569397 Sigma-AldrichStaurosporine, Streptomyces sp.
Staurosporine, CAS 62996-74-, is a cell-permeable, potent, reversible, ATP-competitive inhibitor of protein kinases (IC₅₀ = 7, 20, 1.3, 0.7, & 8.5 nM for PKA, CAMK, MLCK, PKC, & PKG, respectively).
More>> Staurosporine, CAS 62996-74-, is a cell-permeable, potent, reversible, ATP-competitive inhibitor of protein kinases (IC₅₀ = 7, 20, 1.3, 0.7, & 8.5 nM for PKA, CAMK, MLCK, PKC, & PKG, respectively). Less<<Synonyms: MLCK Inhibitor I, PKA Inhibitor II
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
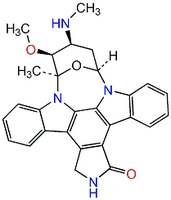
| 62996-74-1 | C₂₈H₂₆N₄O₃ |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 569397-100UG |
|
Plastic ampoule | 100 μg |
|
— | |
| 569397-1MG |
|
Plastic ampoule | 1 mg |
|
— | |
| 569397-250UG |
|
Plastic ampoule | 250 μg |
|
— |
| Description | |
|---|---|
| Overview | A potent, cell-permeable, reversible, ATP-competitive and broad spectrum inhibitor of protein kinases. Inhibits protein kinase A (IC50 = 7 nM), CaM kinase (IC50 = 20 nM), myosin light chain kinase (IC50 = 1.3 nM), protein kinase C (IC50 = 700 pM), and protein kinase G (IC50 = 8.5 nM). Also inhibits platelet aggregation induced by collagen or ADP but has no effect on thrombin-induced platelet aggregation. Induces apoptosis in human malignant glioma cell lines. Arrests normal cells at the G1 checkpoint. A 1 mM (100 µg/214 µl) solution of Staurosporine (Cat. No. 569396) in DMSO is also available. |
| Catalogue Number | 569397 |
| Brand Family | Calbiochem® |
| Synonyms | MLCK Inhibitor I, PKA Inhibitor II |
| Product Information | |
|---|---|
| CAS number | 62996-74-1 |
| ATP Competitive | Y |
| Form | White to pale yellow solid |
| Hill Formula | C₂₈H₂₆N₄O₃ |
| Chemical formula | C₂₈H₂₆N₄O₃ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 569397-100UG | 07790788057855 |
| 569397-1MG | 04055977190465 |
| 569397-250UG | 04055977190496 |
Documentation
Staurosporine, Streptomyces sp. SDS
| Title |
|---|
Staurosporine, Streptomyces sp. Certificates of Analysis
| Title | Lot Number |
|---|---|
| 569397 |
References
| Reference overview |
|---|
| Couldwell, W.T., et al. 1994. FEBS Lett. 345, 43. Nishimura, H. and Simpson, I.A. 1994. Biochem. J. 302, 271. Bruno, S., et al. 1992. Cancer Res. 52, 470. Kiss, Z. and Deli, E. 1992. Biochem. J. 288, 853. Vitale, M.L., et al. 1992. Neuroscience 51, 463. Hoffman, R. and Newland, E.S. 1991. Cancer Chemother. Pharmacol. 28, 102. Oka, S., et al. 1986. Agric. Biol. Chem. 50, 2723. |
Citations
| Title | |
|---|---|
|
|
| Data Sheet | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|