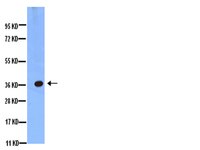
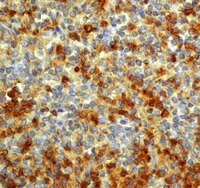
Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis.
Misu, T, et al.
Brain, 130: 1224-34 (2007)
2007
Show Abstract
Neuromyelitis optica (NMO) is an inflammatory and necrotizing disease clinically characterized by selective involvement of the optic nerves and spinal cord. There has been a long controversy as to whether NMO is a variant of multiple sclerosis (MS) or a distinct disease. Recently, an NMO-specific antibody (NMO-IgG) was found in the sera from patients with NMO, and its target antigen was identified as aquaporin 4 (AQP4) water channel protein, mainly expressed in astroglial foot processes. However, the pathogenetic role of the AQP4 in NMO remains unknown. We did an immunohistopathological study on the distribution of AQP4, glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), activated complement C9neo and immunoglobulins in the spinal cord lesions and medulla oblongata of NMO (n = 12), MS (n = 6), brain and spinal infarction (n = 7) and normal control (n = 8). The most striking finding was that AQP4 immunoreactivity was lost in 60 out of a total of 67 acute and chronic NMO lesions (90%), but not in MS plaques. The extensive loss of AQP4 accompanied by decreased GFAP staining was evident, especially in the active perivascular lesions, where immunoglobulins and activated complements were deposited. Interestingly, in those NMO lesions, MBP-stained myelinated fibres were relatively preserved despite the loss of AQP4 and GFAP staining. The areas surrounding the lesions in NMO had enhanced expression of AQP4 and GFAP, which reflected reactive gliosis. In contrast, AQP4 immunoreactivity was well preserved and rather strongly stained in the demyelinating MS plaques, and infarcts were also stained for AQP4 from the very acute phase of necrosis to the chronic stage of astrogliosis. In normal controls, AQP4 was diffusely expressed in the entire tissue sections, but the staining in the spinal cord was stronger in the central grey matter than in the white matter. The present study demonstrated that the immunoreactivities of AQP4 and GFAP were consistently lost from the early stage of the lesions in NMO, notably in the perivascular regions with complement and immunoglobulin deposition. These features in NMO were distinct from those of MS and infarction as well as normal controls, and suggest that astrocytic impairment associated with the loss of AQP4 and humoral immunity may be important in the pathogenesis of NMO lesions. | Immunohistochemistry (Paraffin) | Human | 17405762
 |
Expression of neurodevelopmental markers by cultured porcine neural precursor cells.
Schwartz, Philip H, et al.
Stem Cells, 23: 1286-94 (2005)
2005
Show Abstract
Despite the increasing importance of the pig as a large animal model, little is known about porcine neural precursor cells. To evaluate the markers expressed by these cells, brains were dissected from 60-day fetuses, enzymatically dissociated, and grown in the presence of epidermal growth factor, basic fibroblast growth factor, and platelet-derived growth factor. Porcine neural precursors could be grown as suspended spheres or adherent monolayers, depending on culture conditions. Expanded populations were banked or harvested for analysis using reverse transcription-polymerase chain reaction (RT-PCR), immunocytochemistry, microarrays, and flow cytometry, and results compared with data from analogous human forebrain progenitor cells. Cultured porcine neural precursors widely expressed neural cell adhesion molecule (NCAM), polysialic acid (PSA)-NCAM, vimentin, Ki-67, and Sox2. Minority subpopulations of cells expressed doublecortin, beta-III tubulin, synapsin I, glial fibrillary acidic protein (GFAP), and aquaporin 4 (AQP4) consistent with increased lineage restriction. A human microarray detected porcine transcripts for nogoA (RTN4) and stromal cell-derived factor 1 (SDF1), possibly cyclin D2 and Pbx1, but not CD133, Ki-67, nestin, or nucleostemin. Subsequent RT-PCR showed pig forebrain precursors to be positive for cyclin D2, nucleostemin, nogoA, Pbx1, vimentin, and a faint band for SDF1, whereas no signal was detected for CD133, fatty acid binding protein 7 (FABP7), or Ki-67. Human forebrain progenitor cells were positive for all the genes mentioned. This study shows that porcine neural precursors share many characteristics with their human counterparts and, thus, may be useful in porcine cell transplantation studies potentially leading to the application of this strategy in the setting of nervous system disease and injury. | Immunocytochemistry | Porcine | 16100001
 |