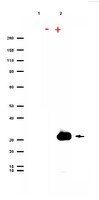
G196 epitope tag system: a novel monoclonal antibody, G196, recognizes the small, soluble peptide DLVPR with high affinity.
Tatsumi K, Sakashita G, Nariai Y, Okazaki K, Kato H, Obayashi E, Yoshida H, Sugiyama K, Park SY, Sekine J, Urano T.
Sci Rep
7
43480
2017
Show Abstract
The recognition specificity of monoclonal antibodies (mAbs) has made mAbs among the most frequently used tools in both basic science research and in clinical diagnosis and therapies. Precise determination of the epitope allows the development of epitope tag systems to be used with recombinant proteins for various purposes. Here we describe a new family of tag derived from the epitope recognized by a highly specific mAb G196. The minimal epitope was identified as the five amino acid sequence Asp-Leu-Val-Pro-Arg. Permutation analysis was used to characterize the binding requirements of mAb G196, and the variable regions of the mAb G196 were identified and structurally analyzed by X-ray crystallography. Isothermal titration calorimetry revealed the high affinity (Kd = 1.25 nM) of the mAb G196/G196-epitope peptide interaction, and G196-tag was used to detect several recombinant cytosolic and nuclear proteins in human and yeast cells. mAb G196 is valuable for developing a new peptide tagging system for cell biology and biochemistry research. | 28266535
 |
The N-terminus and Tudor domains of Sgf29 are important for its heterochromatin boundary formation function.
Kamata K, Goswami G, Kashio S, Urano T, Nakagawa R, Uchida H, Oki M.
J Biochem
155(3)
159-71
2014
Show Abstract
Eukaryotic chromosomes are organized into heterochromatin and euchromatin domains. Heterochromatin domains are transcriptionally repressed and prevented from spreading into neighbouring genes by chromatin boundaries. Previously, we identified 55 boundary-related genes in Saccharomyces cerevisiae. In this study, we describe the characterization of one of these boundary genes, named SGF29, which was previously reported as a component of the SAGA, SLIK, ADA and HAT-A2 complex. A domain analysis of Sgf29 identified two minimal regions that can function as individual boundaries. The N-terminal minimal region comprising amino acids 1-12, which has not been defined as a functional domain, showed stronger boundary formation ability than the C-terminal minimal region comprising amino acids 110-255, which contains Tudor domains. Together with Ada2, Ada3 and Sgf29, which are all components of SAGA, Gcn5 acetylates multiple lysine residues on nucleosomal histone H3, which is associated with an open chromatin structure. However, the results presented in this study suggest that the boundary formation ability of the Sgf29 minimal regions is independent of Gcn5. An in vivo analysis also revealed that Sgf29 and Gcn5 perform distinct functions at native telomere boundary regions on the chromosome. | 24307402
 |
C-terminus of the Sgf73 subunit of SAGA and SLIK is important for retention in the larger complex and for heterochromatin boundary function.
Kamata K, Hatanaka A, Goswami G, Shinmyozu K, Nakayama J, Urano T, Hatashita M, Uchida H, Oki M.
Genes Cells
18(9)
823-37
2013
Show Abstract
The budding yeast Saccharomyces cerevisiae contains active and inactive chromatin separated by boundary domains. Previously, we used genome-wide screening to identify 55 boundary-related genes. Here, we focus on Sgf73, a boundary protein that is a component of the Spt-Ada-Gcn5 acetyltransferase (SAGA) and SLIK (SAGA-like) complexes. These complexes have histone acetyltransferase (HAT) and histone deubiquitinase activity, and Sgf73 is one of the factors necessary to anchor the deubiquitination module. Domain analysis of Sgf73 was carried out, and the minimum region (373-402 aa) essential for boundary function was identified. This minimum region does not include the domain involved in anchoring the deubiquitination module, suggesting that the histone deubiquitinase activity of Sgf73 is not important for its boundary function. Next, Sgf73-mediated boundary function was analyzed in disruption strains in which different protein subunits of the SAGA/SLIK/ADA complexes were deleted. Deletion of ada2, ada3 or gcn5 (a HAT module component) caused complete loss of the boundary function of Sgf73. The importance of SAGA or SLIK complex binding to the boundary function of Sgf73 was also analyzed. Western blot analysis detected both the full-length and truncated forms of Spt7, suggesting that SAGA and SLIK complex formation is important for the boundary function of Sgf73. | 23819448
 |