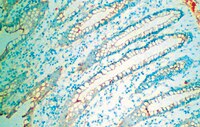
Expression of myoferlin in human airway epithelium and its role in cell adhesion and zonula occludens-1 expression.
Leung, C; Shaheen, F; Bernatchez, P; Hackett, TL
PloS one
7
e40478
2012
Show Abstract
Normal airway epithelial barrier function is maintained by cell-cell contacts which require the translocation of adhesion proteins at the cell surface, through membrane vesicle trafficking and fusion events. Myoferlin and dysferlin, members of the multiple-C2-domain Ferlin superfamily, have been implicated in membrane fusion processes through the induction of membrane curvature. The objectives of this study were to examine the expression of dysferlin and myoferlin within the human airway and determine the roles of these proteins in airway epithelial homeostasis.The expression of dysferlin and myoferlin were evaluated in normal human airway sections by immunohistochemistry, and primary human airway epithelial cells and fibroblasts by immuno blot. Localization of dysferlin and myoferlin in epithelial cells were determined using confocal microscopy. Functional outcomes analyzed included cell adhesion, protein expression, and cell detachment following dysferlin and myoferlin siRNA knock-down, using the human bronchial epithelial cell line, 16HBE.Primary human airway epithelial cells express both dysferlin and myoferlin whereas fibroblasts isolated from bronchi and the parenchyma only express myoferlin. Expression of dysferlin and myoferlin was further localized within the Golgi, cell cytoplasm and plasma membrane of 16HBE cells using confocal micrscopy. Treatment of 16HBE cells with myoferlin siRNA, but not dysferlin siRNA, resulted in a rounded cell morphology and loss of cell adhesion. This cell shedding following myoferlin knockdown was associated with decreased expression of tight junction molecule, zonula occludens-1 (ZO-1) and increased number of cells positive for apoptotic markers Annexin V and propidium iodide. Cell shedding was not associated with release of the innate inflammatory cytokines IL-6 and IL-8.This study demonstrates the heterogeneous expression of myoferlin within epithelial cells and fibroblasts of the respiratory airway. The effect of myoferlin on the expression of ZO-1 in airway epithelial cells indicates its role in membrane fusion events that regulate cell detachment and apoptosis within the airway epithelium. | Western Blotting | Human | 22808170
 |
Refractive lenticule re-implantation after myopic ReLEx: a feasibility study of stromal restoration after refractive surgery in a rabbit model.
Angunawela, RI; Riau, AK; Chaurasia, SS; Tan, DT; Mehta, JS
Investigative ophthalmology & visual science
53
4975-85
2012
Show Abstract
To investigate the potential of refractive lenticule (RL) storage and re-implantation in vivo as a method for reversing RL extraction (ReLEx) and restoring corneal stromal volume.ReLEx [-6.00 diopter (D) correction] was performed on six New Zealand White rabbits in one eye. Each extracted RL was tagged and orientated before storage at -80°C for 28 days. Each RL was then re-implanted autologously in the correct orientation after flap relifting. All animals were monitored for 28 days before being euthanized for immunohistochemical analysis. Unoperated fellow eyes were used as controls. All animals had regular pre- and postoperative slit lamp photography, in vivo confocal microscopy, anterior segment optical coherence tomography (AS-OCT), keratometry, and topography.No intra-operative complications occurred and RL re-implantation was performed without complication. A mild intrastromal haziness was noted on day 3 after re-implantation (corneal haze grade: 2.20 ± 0.45), but corneas were clear on day 28 (0.20 ± 0.27). RL re-implantation restored central corneal thickness, and keratometric and topographic indices to near pre-operative values. Wound healing processes, marked by fibronectin and tenascin, and a few inflammatory cells were present along the re-implanted lenticular interfaces. No myofibroblasts formation, and Ki67- and TUNEL-positive cells were observed in the corneal stroma on postoperative day 28.RL storage and re-implantation is a feasible technique for restoring stromal volume after myopic ReLEx, and may provide a method for restoring tissue in ectatic corneas, or provide an opportunity for further refractive surgery and presbyopic treatment. | | | 22743323
 |
Further expansion of the phenotypic spectrum associated with mutations in ALDH18A1, encoding ?¹-pyrroline-5-carboxylate synthase (P5CS).
David L Skidmore,David Chitayat,Tim Morgan,Alek Hinek,Bjoern Fischer,Aikaterini Dimopoulou,Gino Somers,William Halliday,Susan Blaser,Yenge Diambomba,Edmond G Lemire,Uwe Kornak,Stephen P Robertson
American journal of medical genetics. Part A
155A
2011
Show Abstract
We report on the third case of cutis laxa and progeroid features caused by a homozygous mutation in ALDH18A1 that encodes ?¹-pyrroline-5-carboxylate-synthase (P5CS). This severely affected child, born to consanguineous parents of Pakistani origin, presented with lax, wrinkled and thin skin with dilated and tortuous subcutaneous blood vessels, corneal clouding, and hypotonia. The child had severe global developmental delay and feeding difficulties and died in infancy for an unknown reason. The proband was homozygous for a mutation in ALDH18A1, c.1923?+?1G?>?A which results in the production of two anomalous transcripts that are predicted to encode proteins lacking the catalytic site for the enzyme. The cellular phenotype is characterized by diminished production of collagen types I and III, altered elastin ultrastructure, and diminished cell proliferation of cultured dermal fibroblasts. This severe clinical and cellular phenotype overlaps with a broad group of neurocutaneous syndromes that include cutis laxa type II, wrinkly skin syndrome, de Barsy syndrome, and gerodermia osteodysplastica. The findings presented here emphasize the pleiotropic presentation of this group of conditions and suggest that multiple components of the extracellular matrix are perturbed in these disorders. | | | 21739576
 |
Serum amyloid P ameliorates radiation-induced oral mucositis and fibrosis.
Murray, LA; Kramer, MS; Hesson, DP; Watkins, BA; Fey, EG; Argentieri, RL; Shaheen, F; Knight, DA; Sonis, ST
Fibrogenesis & tissue repair
3
11
2010
Show Abstract
To evaluate the effect of the anti-fibrotic protein serum amyloid P (SAP) on radiation-induced oral mucositis (OM) and fibrosis in a hamster cheek-pouch model.Hamsters received a single dose of radiation (40 Gy) to the left everted cheek pouch to induce significant OM. The protective therapeutic potential of SAP was evaluated using varying dosing regimens. The extent of OM was measured using a validated six-point scoring scheme ranging from 0 (normal tissue, no mucositis) to 5 (complete ulceration). Fibrotic remodeling was also visualized histologically and quantified at later time points using collagen gene expression.SAP treatment attenuated the profile of radiation-induced oral mucositis by delaying the time of onset, reducing the peak value, and enhancing the resolution of injury. The peak mucositis score was reduced by approximately 0.5 grade in SAP-treated animals. The number of animal days with a score of greater than /= 3 was reduced by 48% in the SAP-treated group, compared with the saline control group (P less than 0.01). SAP also inhibited the extent of tissue remodeling and decreased radiation-induced increases in myofibroblast number. Attenuated collagen deposition and gene expression was also observed in the cheek pouches of hamsters treated with SAP at both 16 and 28 days post-radiation.SAP treatment significantly attenuated radiation-induced injury. In particular, SAP attenuated the severity of OM and inhibited pathogenic remodeling. This suggests that SAP may be a useful therapy for the palliation of side effects observed during treatment for head and neck cancer. | | | 20602770
 |
Selegiline is an efficient and potent inducer for bone marrow stromal cell differentiation into neuronal phenotype.
Mohammad Taghi Ghorbanian,Taki Tiraihi,Seyed A Mesbah-Namin,Yaghoub Fathollahi
Neurological research
32
2010
Show Abstract
Bone marrow stromal cells (BMSCs) were documented as a feasible candidate for cell therapy. Several protocols with one or more steps, using different chemicals, were used for inducing BMSCs differentiation into neuronal phenotype. Many of these chemicals were reported to be of mutagenic, teratogenic or carcinogenic properties. The purpose of this work was to evaluate the neuronal inductivity of selegiline to BMSCs. | | | 19422735
 |
BMP-7 does not protect against bleomycin-induced lung or skin fibrosis.
Murray, LA; Hackett, TL; Warner, SM; Shaheen, F; Argentieri, RL; Dudas, P; Farrell, FX; Knight, DA
PloS one
3
e4039
2008
Show Abstract
Bone morphogenic protein (BMP)-7 is a member of the BMP family which are structurally and functionally related, and part of the TGFbeta super family of growth factors. BMP-7 has been reported to inhibit renal fibrosis and TGFbeta1-induced epithelial-mesenchymal transition (EMT), in part through negative interactions with TGFbeta1 induced Smad 2/3 activation. We utilized in vivo bleomycin-induced fibrosis models in the skin and lung to determine the potential therapeutic effect of BMP-7. We then determined the effect of BMP-7 on TGFbeta1-induced EMT in lung epithelial cells and collagen production by human lung fibroblasts. We show that BMP-7 did not affect bleomycin-induced fibrosis in either the lung or skin in vivo; had no effect on expression of pro-fibrotic genes by human lung fibroblasts, either at rest or following exposure to TGFbeta1; and did not modulate TGFbeta1-induced EMT in human lung epithelial cells. Taken together our data indicates that BMP-7 has no anti-fibrotic effect in lung or skin fibrosis either in vivo or in vitro. This suggests that the therapeutic options for BMP-7 may be confined to the renal compartment. Full Text Article | | | 19112509
 |
Proteolytic digest derived from bovine Ligamentum Nuchae stimulates deposition of new elastin-enriched matrix in cultures and transplants of human dermal fibroblasts.
Aleksander Hinek, Yanting Wang, Kela Liu, Thomas F Mitts, Felipe Jimenez
Journal of dermatological science
39
155-66
2005
Show Abstract
BACKGROUND: Diverse topical products and injectable fillers used for correcting facial wrinkles induce rather short-lived effects because they target replacement of dermal collagen and hyaluronan, matrix components of limited biologic durability. OBJECTIVE: Present studies were aimed at stimulation of fully differentiated human dermal fibroblasts to resume deposition of new extracellular matrix rich of elastin, the most durable and metabolically inert component of dermal ECM. METHODS: We have created a novel proteolytic digest of bovine ligamentum nuchae (ProK-60), and tested its potential biological effect on dermal fibroblasts derived from females of different ages. Northern blots, quantitative immunohistochemistry and metabolic assays were used to assess effects of ProK-60 on proliferation and matrix production in primary cultures of dermal fibroblasts, in cultures of skin explants and after implantation of stimulated fibroblasts into the skin of athymic nude mice. RESULTS: ProK-60 increased proliferation (25-30%) of cultured dermal fibroblasts and significantly enhanced their production of new elastic fibers (>250%) and collagen fibers (100%). These effects were mostly mediated by stimulation of cellular elastin receptor. In contrast, ProK-60 inhibited production of fibronectin (-30%) and chondroitin sulfate proteoglycans (-50%). ProK-60 also activated proliferation of dermal fibroblasts, mostly derived from the stratum basale and induced deposition of elastic fibers in cultures of skin explants. Moreover, human fibroblasts pre-treated with ProK-60 produced abundant elastic fibers after their injection into the skin of athymic nude mice. CONCLUSION: The described biological effects of ProK-60, including its unique elastogenic property, encourage use of this compound in cosmetic formulations stimulating rejuvenation of aged skin. | | | 15925490
 |
Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis.
Surendran, K; Schiavi, S; Hruska, KA
Journal of the American Society of Nephrology : JASN
16
2373-84
2005
Show Abstract
beta-Catenin functions as a transducer of Wnt signals to the nucleus, where it interacts with the T cell factor (TCF) family of DNA binding proteins to regulate gene expression. On the basis of the genes regulated by beta-catenin and TCF in various biologic settings, two predicted functions of beta-catenin/TCF-dependent transcription are to mediate the loss of epithelial polarity and to promote fibroblast activities, such as the increased synthesis of fibronectin during chronic renal disease. These predictions were tested by determination of the expression and function of an inhibitor of Wnt signaling, secreted frizzled-related protein 4 (sFRP4), during renal tubular epithelial injury initiated by unilateral ureteral obstruction (UUO). Despite increased sFRP4 gene expression in perivascular regions of injured kidneys, total sFRP4 protein levels decreased after injury. The decreased sFRP4 protein levels after UUO accompanied increased Wnt-dependent beta-catenin signaling in tubular epithelial and interstitial cells, along with increased expression of markers of fibrosis. Administration of recombinant sFRP4 protein caused a reduction in tubular epithelial beta-catenin signaling and suppressed the progression of renal fibrosis, as evidenced by a partial maintenance of E-cadherin mRNA expression and a reduction in the amount of fibronectin and alpha-smooth muscle actin proteins. Furthermore, recombinant sFRP4 reduced the number of myofibroblasts, a central mediator of fibrosis. It is concluded that beta-catenin signaling is activated in tubular epithelial and interstitial cells after renal injury, and recombinant sFRP4 can interfere with epithelial de-differentiation and with fibroblast differentiation and function during progression of renal fibrosis. | Western Blotting | | 15944336
 |
Kynurenate production by cultured human astrocytes.
C Kiss, G Ceresoli-Borroni, P Guidetti, C L Zielke, H R Zielke, R Schwarcz
Journal of neural transmission (Vienna, Austria : 1996)
110
1-14
2003
Show Abstract
In the rodent brain, astrocytes are known to be the primary source of kynurenate (KYNA), an endogenous antagonist of both the glycine(B) and the alpha7 nicotinic acetylcholine receptor. In the present study, primary human astrocytes were used to examine the characteristics and regulation of de novo KYNA synthesis in vitro. To this end, cells were exposed to KYNA's bioprecursor L-kynurenine, and newly formed KYNA was recovered from the extracellular milieu. The production of KYNA was stereospecific and rose with increasing L-kynurenine concentrations, reaching a plateau in the high microM range. In an analogous experiment, astrocytes also readily produced and liberated the potent, specific glycine(B) receptor antagonist 7-chlorokynurenate from L-4-chlorokynurenine. KYNA synthesis was dose-dependently reduced by L-leucine or L-phenylalanine, two amino acids that compete with L-kynurenine for cellular uptake, and by aminooxyacetate, a non-specific aminotransferase inhibitor. In contrast, KYNA formation was stimulated by 5 mM pyruvate or oxaloacetate, which act as co-substrates of the transamination reaction. Aglycemic or depolarizing (50 mM KCl or 100 microM veratridine) conditions had no effect on KYNA synthesis. Subsequent studies using tissue homogenate showed that both known cerebral kynurenine aminotransferases (KAT I and KAT II) are present in astrocytes, but that KAT II appears to be singularly responsible for KYNA formation under physiological conditions. Taken together with previous results, these data suggest that very similar mechanisms control KYNA synthesis in the rodent and in the human brain. These regulatory events are likely to influence the neuromodulatory effects of astrocyte-derived KYNA in the normal and diseased human brain. | | | 12541009
 |
Retroviral gene therapy vectors for prevention of excimer laser-induced corneal haze.
Ashley Behrens, Erlinda M Gordon, Li Li, Peng X Liu, Zhenhai Chen, Hongjun Peng, Laurie La Bree, W French Anderson, Frederick L Hall, Peter J McDonnell
Investigative ophthalmology visual science
43
968-77
2002
Show Abstract
PURPOSE: To determine the in vivo efficacy and safety of a retroviral vector bearing an antiproliferative dominant negative mutant cyclin G1 (dnG1) construct, when used for the prevention of corneal haze after phototherapeutic keratectomy (PTK). METHODS: For in vivo efficacy studies, a 6-mm-diameter, 150-microm-deep transepithelial PTK, performed with a clinical 193-nm ArF excimer laser (VISX Star2, Santa Clara, CA) was performed on the left eyes of 20 adult New Zealand White rabbits. The surgically altered eyes were subsequently treated with eye drops containing: a retroviral vector bearing a dnG1 construct (dnG1; n = 7), a control retroviral vector (null vector) bearing only the neomycin resistance, neo(r), gene (n = 7), or a retroviral vector bearing an antisense cyclin G1 (aG1) construct (n = 6). The time of closure of the corneal epithelial defect was monitored daily with fluorescein staining. Corneal haze was evaluated before surgery and at 2, 3, and 4 weeks after surgery, with a digital imaging system. Biodistribution studies for detection of potential vector dissemination to nontarget organs were conducted by PCR-based assay. RESULTS: The re-epithelialization rate was similar among treatment groups, with complete closure of the corneal epithelial defect at 72 hours (P > 0.05). Significant corneal haze developed in the null and aG1 vector-treated groups (P or= 0.05) at 3 to 4 weeks after PTK. In contrast, development of corneal haze was inhibited in the dnG1 vector-treated group when compared with the null vector-treated group (P 0.05). In parallel, a dramatic reduction to complete abrogation of abnormal extracellular matrix production was noted in the dnG1 vector-treated corneas when compared with the null and aG1 vector-treated groups. Biodistribution studies showed no evidence of vector dissemination in neighboring and distant organs. CONCLUSIONS: At therapeutic doses, eye drop application of the dnG1 retroviral vector is safe and effective in inhibiting development of corneal haze after PTK in rabbits. | | | 11923236
 |