An intrinsically disordered region of methyl-CpG binding domain protein 2 (MBD2) recruits the histone deacetylase core of the NuRD complex.
Desai, MA; Webb, HD; Sinanan, LM; Scarsdale, JN; Walavalkar, NM; Ginder, GD; Williams, DC
Nucleic acids research
43
3100-13
2015
Show Abstract
The MBD2-NuRD (Nucleosome Remodeling and Deacetylase) complex is an epigenetic reader of DNA methylation that regulates genes involved in normal development and neoplastic diseases. To delineate the architecture and functional interactions of the MBD2-NuRD complex, we previously solved the structures of MBD2 bound to methylated DNA and a coiled-coil interaction between MBD2 and p66α that recruits the CHD4 nucleosome remodeling protein to the complex. The work presented here identifies novel structural and functional features of a previously uncharacterized domain of MBD2 (MBD2IDR). Biophysical analyses show that the MBD2IDR is an intrinsically disordered region (IDR). However, despite this inherent disorder, MBD2IDR increases the overall binding affinity of MBD2 for methylated DNA. MBD2IDR also recruits the histone deacetylase core components (RbAp48, HDAC2 and MTA2) of NuRD through a critical contact region requiring two contiguous amino acid residues, Arg(286) and Leu(287). Mutating these residues abrogates interaction of MBD2 with the histone deacetylase core and impairs the ability of MBD2 to repress the methylated tumor suppressor gene PRSS8 in MDA-MB-435 breast cancer cells. These findings expand our knowledge of the multi-dimensional interactions of the MBD2-NuRD complex that govern its function. | | 25753662
 |
Histone methyltransferase MMSET/NSD2 alters EZH2 binding and reprograms the myeloma epigenome through global and focal changes in H3K36 and H3K27 methylation.
Popovic, R; Martinez-Garcia, E; Giannopoulou, EG; Zhang, Q; Zhang, Q; Ezponda, T; Shah, MY; Zheng, Y; Will, CM; Small, EC; Hua, Y; Bulic, M; Jiang, Y; Carrara, M; Calogero, RA; Kath, WL; Kelleher, NL; Wang, JP; Elemento, O; Licht, JD
PLoS genetics
10
e1004566
2014
Show Abstract
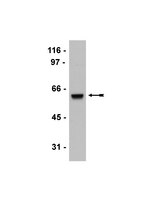
Overexpression of the histone methyltransferase MMSET in t(4;14)+ multiple myeloma patients is believed to be the driving factor in the pathogenesis of this subtype of myeloma. MMSET catalyzes dimethylation of lysine 36 on histone H3 (H3K36me2), and its overexpression causes a global increase in H3K36me2, redistributing this mark in a broad, elevated level across the genome. Here, we demonstrate that an increased level of MMSET also induces a global reduction of lysine 27 trimethylation on histone H3 (H3K27me3). Despite the net decrease in H3K27 methylation, specific genomic loci exhibit enhanced recruitment of the EZH2 histone methyltransferase and become hypermethylated on this residue. These effects likely contribute to the myeloma phenotype since MMSET-overexpressing cells displayed increased sensitivity to EZH2 inhibition. Furthermore, we demonstrate that such MMSET-mediated epigenetic changes require a number of functional domains within the protein, including PHD domains that mediate MMSET recruitment to chromatin. In vivo, targeting of MMSET by an inducible shRNA reversed histone methylation changes and led to regression of established tumors in athymic mice. Together, our work elucidates previously unrecognized interplay between MMSET and EZH2 in myeloma oncogenesis and identifies domains to be considered when designing inhibitors of MMSET function. | Western Blotting | 25188243
 |
RanBP9 aggravates synaptic damage in the mouse brain and is inversely correlated to spinophilin levels in Alzheimer's brain synaptosomes.
Palavicini, JP; Wang, H; Bianchi, E; Xu, S; Rao, JS; Kang, DE; Lakshmana, MK
Cell death & disease
4
e667
2013
Show Abstract
We previously demonstrated that overexpression of RanBP9 led to enhanced Aβ generation in a variety of cell lines and primary neuronal cultures, and subsequently, we confirmed increased amyloid plaque burden in a mouse model of Alzheimer's disease (AD). In the present study, we found striking reduction of spinophilin protein levels when RanBP9 is overexpressed. At 12 months of age, we found spinophilin levels reduced by 70% (Pless than 0.001) in the cortex of APΔE9/RanBP9 mice compared with that in wild-type (WT) controls. In the hippocampus, the spinophilin levels were reduced by 45% (Pless than 0.01) in the APΔE9/RanBP9 mice. Spinophilin immunoreactivity was also reduced by 22% (Pless than 0.01) and 12% (Pless than 0.05) in the cortex of APΔE9/RanBP9 and APΔE9 mice, respectively. In the hippocampus, the reductions were 27% (Pless than 0.001) and 14% (Pless than 0.001) in the APΔE9/RanBP9 and APΔE9 mice, respectively. However, in the cerebellum, spinophilin levels were not altered in either APΔE9 or APΔE9/RanBP9 mice. Additionally, synaptosomal functional integrity was reduced under basal conditions by 39% (Pless than 0.001) in the APΔE9/RanBP9 mice and ~23% (Pless than 0.001) in the APΔE9 mice compared with that in WT controls. Under ATP- and KCl-stimulated conditions, we observed higher mitochondrial activity in the WT and APΔE9 mice, but lower in the APΔE9/RanBP9 mice. Significantly, we confirmed the inverse relationship between RanBP9-N60 and spinophilin in the synaptosomes of Alzheimer's brains. More importantly, both APΔE9 and APΔE9/RanBP9 mice showed impaired learning and memory skills compared to WT controls. These data suggest that RanBP9 might play a crucial role in the loss of spines and synapses in AD. | | 23764848
 |
The human RVB complex is required for efficient transcription of type I interferon-stimulated genes.
Gnatovskiy, L; Mita, P; Levy, DE
Molecular and cellular biology
33
3817-25
2013
Show Abstract
Type I interferons (IFNs) stimulate transcription through a latent heterotrimeric transcription factor composed of tyrosine-phosphorylated STAT1 and STAT2 and the DNA binding partner IRF9, with STAT2 contributing a critical transactivation domain. Human RVB1 and RVB2, which are highly conserved AAA(+) ATP binding proteins contained in chromatin-remodeling complexes such as Ino80, SNF2-related CBP activator protein (SRCAP), and Tip60/NuA4, interacted with the transactivation domain of STAT2 in the nuclei of IFN-stimulated cells. RNA interference (RNAi) experiments demonstrated that RVB proteins were required for robust activation of IFN-α-stimulated genes (ISGs). The requirement for RVB proteins was specific to IFN-α/STAT2 signaling; transcription of tumor necrosis factor alpha (TNF-α)- and IFN-γ-driven genes was not affected by RVB1 depletion. Using RNAi-based depletion, we assessed the involvement of catalytic subunits of the RVB-containing Tip60, BRD8, Ino80, SRCAP, and URI complexes. No component other than RVB1/2 was uniquely required for ISG induction, suggesting that RVB1/2 functions as part of an as yet unidentified complex. Chromatin immunoprecipitation assays indicated that RVB1/2 was required for recruitment of RNA polymerase II (Pol II) to ISG promoters but was dispensable for STAT2 recruitment to chromatin. We hypothesize that an RVB1/2 chromatin-remodeling complex is required for efficient Pol II recruitment and initiation at ISG promoters and is recruited through interaction with the STAT2 transactivation domain. | | 23878400
 |
Physical interaction between MYCN oncogene and polycomb repressive complex 2 (PRC2) in neuroblastoma: functional and therapeutic implications.
Corvetta, D; Chayka, O; Gherardi, S; D'Acunto, CW; Cantilena, S; Valli, E; Piotrowska, I; Perini, G; Sala, A
The Journal of biological chemistry
288
8332-41
2013
Show Abstract
CLU (clusterin) is a tumor suppressor gene that we have previously shown to be negatively modulated by the MYCN proto-oncogene, but the mechanism of repression was unclear. Here, we show that MYCN inhibits the expression of CLU by direct interaction with the non-canonical E box sequence CACGCG in the 5'-flanking region. Binding of MYCN to the CLU gene induces bivalent epigenetic marks and recruitment of repressive proteins such as histone deacetylases and Polycomb members. MYCN physically binds in vitro and in vivo to EZH2, a component of the Polycomb repressive complex 2, required to repress CLU. Notably, EZH2 interacts with the Myc box domain 3, a segment of MYC known to be essential for its transforming effects. The expression of CLU can be restored in MYCN-amplified cells by epigenetic drugs with therapeutic results. Importantly, the anticancer effects of the drugs are ablated if CLU expression is blunted by RNA interference. Our study implies that MYC tumorigenesis can be effectively antagonized by epigenetic drugs that interfere with the recruitment of chromatin modifiers at repressive E boxes of tumor suppressor genes such as CLU. | Western Blotting | 23362253
 |
Regulation of Gγ-globin gene by ATF2 and its associated proteins through the cAMP-response element.
Liu, L; Karmakar, S; Dhar, R; Mahajan, M; Choudhury, A; Weissman, S; Pace, BS
PloS one
8
e78253
2013
Show Abstract
The upstream Gγ-globin cAMP-response element (G-CRE) plays an important role in regulating Gγ-globin expression through binding of ATF2 and its DNA-binding partners defined in this study. ATF2 knockdown resulted in a significant reduction of γ-globin expression accompanied by decreased ATF2 binding to the G-CRE. By contrast, stable ATF2 expression in K562 cells increased γ-globin transcription which was reduced by ATF2 knockdown. Moreover, a similar effect of ATF2 on γ-globin expression was observed in primary erythroid progenitors. To understand the role of ATF2 in γ-globin expression, chromatographically purified G-CRE/ATF2-interacting proteins were subjected to mass spectrometry analysis; major binding partners included CREB1, cJun, Brg1, and histone deacetylases among others. Immunoprecipitation assays demonstrated interaction of these proteins with ATF2 and in vivo GCRE binding in CD34(+) cells undergoing erythroid differentiation which was correlated with γ-globin expression during development. These results suggest synergism between developmental stage-specific recruitments of the ATF2 protein complex and expression of γ-globin during erythropoiesis. Microarray studies in K562 cells support ATF2 plays diverse roles in hematopoiesis and chromatin remodeling. | | 24223142
 |
Stress-induced epigenetic transcriptional memory of acetylcholinesterase by HDAC4.
Sailaja, BS; Cohen-Carmon, D; Zimmerman, G; Soreq, H; Meshorer, E
Proceedings of the National Academy of Sciences of the United States of America
109
E3687-95
2012
Show Abstract
Stress induces long-lasting changes in neuronal gene expression and cholinergic neurotransmission, but the underlying mechanism(s) are incompletely understood. Here, we report that chromatin structure and histone modifications are causally involved in this transcriptional memory. Specifically, the AChE gene encoding the acetylcholine-hydrolyzing enzyme acetylcholinesterase is known to undergo long-lasting transcriptional and alternative splicing changes after stress. In mice subjected to stress, we identified two alternative 5' exons that were down-regulated after stress in the hippocampus, accompanied by reduced acetylation and elevated trimethylation of H3K9 at the corresponding promoter. These effects were reversed completely by daily administration of the histone deacetylase (HDAC) inhibitor sodium butyrate for 1 wk after stress. H3K9 hypoacetylation was associated with a selective, sodium butyrate-reversible promoter accumulation of HDAC4. Hippocampal suppression of HDAC4 in vivo completely abolished the long-lasting AChE-related and behavioral stress effects. Our findings demonstrate long-lasting stress-inducible changes in AChE's promoter choices, identify the chromatin changes that support this long-term transcriptional memory, and reveal HDAC4 as a mediator of these effects in the hippocampus. | | 23236169
 |
Transcription factor Foxp3 and its protein partners form a complex regulatory network.
Rudra, D; deRoos, P; Chaudhry, A; Niec, RE; Arvey, A; Samstein, RM; Leslie, C; Shaffer, SA; Goodlett, DR; Rudensky, AY
Nature immunology
13
1010-9
2012
Show Abstract
The transcription factor Foxp3 is indispensible for the differentiation and function of regulatory T cells (T(reg) cells). To gain insights into the molecular mechanisms of Foxp3-mediated gene expression, we purified Foxp3 complexes and explored their composition. Biochemical and mass-spectrometric analyses revealed that Foxp3 forms multiprotein complexes of 400-800 kDa or larger and identified 361 associated proteins, ∼30% of which were transcription related. Foxp3 directly regulated expression of a large proportion of the genes encoding its cofactors. Some transcription factor partners of Foxp3 facilitated its expression. Functional analysis of the cooperation of Foxp3 with one such partner, GATA-3, provided additional evidence for a network of transcriptional regulation afforded by Foxp3 and its associates to control distinct aspects of T(reg) cell biology. | | 22922362
 |
CBX7 is a tumor suppressor in mice and humans.
Forzati, F; Federico, A; Pallante, P; Abbate, A; Esposito, F; Malapelle, U; Sepe, R; Palma, G; Troncone, G; Scarfò, M; Arra, C; Fedele, M; Fusco, A
The Journal of clinical investigation
122
612-23
2012
Show Abstract
The CBX7 gene encodes a polycomb group protein that is known to be downregulated in many types of human cancers, although the role of this protein in carcinogenesis remains unclear. To shed light on this issue, we generated mice null for Cbx7. Mouse embryonic fibroblasts derived from these mice had a higher growth rate and reduced susceptibility to senescence compared with their WT counterparts. This was associated with upregulated expression of multiple cell cycle components, including cyclin E, which is known to play a key role in lung carcinogenesis in humans. Adult Cbx7-KO mice developed liver and lung adenomas and carcinomas. In in vivo and in vitro experiments, we demonstrated that CBX7 bound to the CCNE1 promoter in a complex that included HDAC2 and negatively regulated CCNE1 expression. Finally, we found that the lack of CBX7 protein expression in human lung carcinomas correlated with CCNE1 overexpression. These data suggest that CBX7 is a tumor suppressor and that its loss plays a key role in the pathogenesis of cancer. | Immunoprecipitation | 22214847
 |
A transcriptional repressor co-regulatory network governing androgen response in prostate cancers.
Chng, KR; Chang, CW; Tan, SK; Yang, C; Hong, SZ; Sng, NY; Cheung, E
The EMBO journal
31
2810-23
2012
Show Abstract
Transcriptional corepressors are frequently aberrantly over-expressed in prostate cancers. However, their crosstalk with the Androgen receptor (AR), a key player in prostate cancer development, is unclear. Using ChIP-Seq, we generated extensive global binding maps of AR, ERG, and commonly over-expressed transcriptional corepressors including HDAC1, HDAC2, HDAC3, and EZH2 in prostate cancer cells. Surprisingly, our results revealed that ERG, HDACs, and EZH2 are directly involved in androgen-regulated transcription and wired into an AR centric transcriptional network via a spectrum of distal enhancers and/or proximal promoters. Moreover, we showed that similar to ERG, these corepressors function to mediate repression of AR-induced transcription including cytoskeletal genes that promote epithelial differentiation and inhibit metastasis. Specifically, we demonstrated that the direct suppression of Vinculin expression by ERG, EZH2, and HDACs leads to enhanced invasiveness of prostate cancer cells. Taken together, our results highlight a novel mechanism by which, ERG working together with oncogenic corepressors including HDACs and the polycomb protein, EZH2, could impede epithelial differentiation and contribute to prostate cancer progression, through directly modulating the transcriptional output of AR. | Western Blotting | 22531786
 |