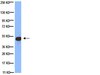
Identification of novel CD8+ T cell epitopes in human herpesvirus 6B U11 and U90.
Halawi, M; Khan, N; Blake, N
Immunity, inflammation and disease
3
118-31
2015
Show Abstract
Human herpesvirus 6B (HHV6B) infects over 90% of the population, and normally establishes a latent infection, where episodes of reactivation are asymptomatic. However, in immunocompromised patients HHV6B reactivation is associated with high morbidity and mortality. Cellular immunotherapy has been utilised against other herpesvirus in immunocompromised settings. However, limited information on the immune response against HHV6B has hampered the development of immunotherapy for HHV6B-driven disease. In this study, we have analysed the cellular immune response against four HHV6B antigens in a panel of 30 healthy donors. We show that the base-line level of T cell reactivity in peripheral blood is very low to undetectable. A short-term reactivation step enabled expansion of T cell responses, and all donors responded to at least 1 antigen, but more commonly 3 or 4. A hierarchy of immunogenicity was determined with antigens U90 and U54 being co-dominant, followed by U11 and U39. Putative CD8+ T cell epitopes were mapped to U90 and U11, predicted to be presented in the context of HLA-A1, A29, B39 and C6. T cells reactive against these novel epitopes were able to recognise virus-infected cells. Our data is supportive of the application and on-going development of T cell immunotherapy against HHVB-driven disease in the immunocompromised host. | 26029371
 |
Immunotherapeutic strategies to prevent and treat human herpesvirus 6 reactivation after allogeneic stem cell transplantation.
Gerdemann, U; Keukens, L; Keirnan, JM; Katari, UL; Nguyen, CT; de Pagter, AP; Ramos, CA; Kennedy-Nasser, A; Gottschalk, SM; Heslop, HE; Brenner, MK; Rooney, CM; Leen, AM
Blood
121
207-18
2013
Show Abstract
Human herpesvirus (HHV) 6 causes substantial morbidity and mortality in the immunocompromised host and has no approved therapy. Adoptive transfer of virus specific T cells has proven safe and apparently effective as prophylaxis and treatment of other virus infections in immunocompromised patients; however, extension to subjects with HHV6 has been hindered by the paucity of information on targets of cellular immunity. We now characterize the cellular immune response from 20 donors against 5 major HHV6B antigens predicted to be immunogenic and define a hierarchy of immunodominance of antigens based on the frequency of responding donors and the magnitude of the T-cell response. We identified specific epitopes within these antigens and expanded the HHV6 reactive T cells using a GMP-compliant protocol. The expanded population comprised both CD4(+) and CD8(+) T cells that were able to produce multiple effector cytokines and kill both peptide-loaded and HHV6B wild-type virus-infected target cells. Thus, we conclude that adoptive T-cell immunotherapy for HHV6 is a practical objective and that the peptide and epitope tools we describe will allow such cells to be prepared, administered, and monitored in human subjects. | 23152545
 |
Development of a new quantitative real-time HHV-6-PCR and monitoring of HHV-6 DNAaemia after liver transplantation.
Teemu Karlsson,Laura Mannonen,Raisa Loginov,Maija Lappalainen,Krister Höckerstedt,Irmeli Lautenschlager
Journal of virological methods
181
2012
Show Abstract
A quantitative HHV-6 PCR (qPCR) assay was developed and compared to an in-house qualitative PCR and to the commercial quantitative Argene CMV, HHV6, 7, 8 R-gene™ test. Clinical specimens consisting of 127 whole blood and 57 cerebrospinal fluid (CSF) specimens were tested using the two qPCRs and the qualitative PCR in parallel. When the qualitative PCR was used as a gold standard, the sensitivities of the qPCRs for the blood samples were 86% for the in-house qPCR and 76% for the Argene's test and the specificities were 96% and 92%, respectively. With CSF specimens the sensitivities were 92% and 80% and the specificities 98% and 82%, respectively. Furthermore, the two qPCRs were compared in the monitoring of liver transplant patients and retrospectively correlated to HHV-6 antigenaemia. In total, 223 blood specimens were tested. HHV-6 antigenaemia had been found in 21/36 (58%) patients and HHV-6 DNAaemia was demonstrated in 18/36 (50%). Viral loads by the in-house test varied from 280 to 19700 copies/ml (median 1200) and by Argene's test from 120 to 24070 copies/ml (median 458). The correlation of viral loads between the two qPCRs was good (R=0.94, p<0.01). The new in-house test was found to be reliable for the detection and quantitation of HHV-6 DNA in clinical specimens. | 22301197
 |
Development of a human herpesvirus 6 species-specific immunoblotting assay.
Higashimoto, Y; Ohta, A; Nishiyama, Y; Ihira, M; Sugata, K; Asano, Y; Peterson, DL; Ablashi, DV; Lusso, P; Yoshikawa, T
Journal of clinical microbiology
50
1245-51
2012
Show Abstract
In order to assess the full spectrum of human herpesvirus 6A (HHV-6A)- and HHV-6B-associated diseases, we sought to develop an HHV-6 species-specific serological assay based on immunoblot analysis. The immunodominant proteins encoded by open reading frame U11, p100 for HHV-6A (strain U1102) and 101K for HHV-6B (strain Z29), were selected to generate virus species-specific antigens. Recombinant p100 and 101K were produced in a prokaryotic expression system. The expression of these proteins was confirmed by using anti-His tag and 101K-specific monoclonal antibodies. HHV-6 species-specific antibodies were detected by immunoblotting in patient sera. Eighty-seven serum samples obtained from various subjects were utilized to determine the reliability of the method for clinical use. Ten of twelve exanthem subitum convalescent-phase sera reacted exclusively with 101K, whereas none of twelve acute-phase sera reacted with either protein. Two of three sera collected from HHV-6A-infected patients reacted with p100 and 101K. Although all five acute and convalescent-phase sera obtained from transplant recipients reacted exclusively with 101K, two of six convalescent-phase sera obtained from patients with drug-induced hypersensitivity syndrome reacted with both p100 and 101K. Of 38 sera obtained from healthy adults, 31 were positive for 101K antibody, while 4 reacted with both proteins. However, PCR analysis of peripheral blood mononuclear cells and saliva from these subjects did not detect HHV-6A DNA. In conclusion, this novel serological assay based on immunoblot analysis using recombinant HHV-6A p100 and HHV-6B 101K allowed us to discriminate between HHV-6A- and HHV-6B-specific antibodies. | 22278837
 |
First analysis of human herpesvirus 6T-cell responses: Specific boosting after HHV6 reactivation in stem cell transplantation recipients.
A P J de Pagter,J J Boelens,J Scherrenburg,T Vroom-de Blank,K Tesselaar,N Nanlohy,E A M Sanders,R Schuurman,D van Baarle
Clinical immunology (Orlando, Fla.)
144
2012
Show Abstract
Early human herpesvirus 6 (HHV6) reactivation after hematopoietic stem cell transplantation (HSCT) is associated with poor survival. We characterized HHV6 immuneresponses in HSCT patients during lymphopenia. Prospectively, HHV6 DNA-load was measured weekly by realtime-PCR. Numbers of IFNγ-producing HHV6-T-cells were retrospectively determined by enzyme-linked immunospot assay 2months after HSCT. HHV6-specific T-cell proliferative capacity was analyzed with a newly developed assay using antigen-presenting autologous HHV6-infected PBMC. Fifty-six patients were included (median age 4.6years; range 0.2-21.2years). HHV6-reactivation occurred in 29/56 (52%) patients with a median time of 14 (range 1-41) days after HSCT. The median number of IFN-γ producing HHV6-specific T-cells at 2months and the HHV6-specific CD8+ T-cell proliferative capacity at 6months after HSCT was increased after HHV6-reactivation compared to non-reactivating patients (P=0.006 and p=0.019). In conclusion, HHV6-specific immuneresponses can be initiated during lymphopenia early after HSCT, which implicates a potential window for development of HHV6-specific (immuno)therapy. | 22820131
 |
Simultaneous monitoring of CMV and human herpesvirus 6 infections and diseases in liver transplant patients: one-year follow-up.
Costa, FA; Soki, MN; Andrade, PD; Bonon, SH; Thomasini, RL; Sampaio, AM; Ramos, Mde C; Rossi, CL; Cavalcanti, TC; Boin, Ide F; Leonard, M; Leonard, LS; Stucchi, RB; Costa, SC
Clinics (São Paulo, Brazil)
66
949-53
2011
Show Abstract
The aim of this study was to simultaneously monitoring cytomegalovirus and human herpesvirus 6 active infections using nested-polymerase chain reaction and, together with clinical findings, follow the clinical status of patients undergoing liver transplant.The human β-herpesviruses, including cytomegalovirus and human herpesvirus 6, are ubiquitous among human populations. Active infections of human herpesvirus 6 and cytomegalovirus are common after liver transplantation, possibly induced and facilitated by allograft rejection and immunosuppressive therapy. Both viruses affect the success of the transplant procedure.Thirty patients submitted to liver transplant at the Liver Transplant Unit, at the Gastro Center, State University of Campinas, SP, Brazil, were studied prospectively from six months to one year, nested-polymerase chain reaction for cytomegalovirus and human herpesvirus 6 DNA detections. Two or more consecutive positive nested-polymerase chain reaction were considered indicative of active infection.Active infection by cytomegalovirus was detected in 13/30 (43.3%) patients, median time to first cytomegalovirus detection was 29 days after transplantation (range: 0-99 days). Active infection by human herpesvirus 6 was detected in 12/30 (40%) patients, median time to first human herpesvirus 6 detection was 23.5 days after transplantation (range: 0-273 days). The time-related appearance of each virus was not statistically different (p = 0.49). Rejection of the transplanted liver was observed in 16.7% (5/30) of the patients. The present analysis showed that human herpesvirus 6 and/or cytomegalovirus active infections were frequent in liver transplant recipients at our center.Few patients remain free of betaherpesviruses after liver transplantation. Most patients presenting active infection with more than one virus were infected sequentially and not concurrently. Nested-polymerase chain reaction can be considered of limited value for clinically monitoring cytomegalovirus and human herpesvirus 6. | 21808857
 |
Human herpesvirus 6 and cytomegalovirus in ileocolonic mucosa in inflammatory bowel disease.
Taina Sipponen,Ulla Turunen,Irmeli Lautenschlager,Urpo Nieminen,Johanna Arola,Leena Halme
Scandinavian journal of gastroenterology
46
2011
Show Abstract
Reactivation of a latent cytomegalovirus (CMV) may occur in inflammatory bowel disease (IBD). Data of human herpesvirus 6 (HHV-6)--a close relative to CMV--in active IBD are scarce. The aim of this study was to detect HHV-6 and CMV antigens in the mucosa of active and inactive IBD. | 21879802
 |
Human herpes virus 6 encephalomyelitis after bone marrow transplantation: report of an autopsy case.
Masayuki Shintaku,Daita Kaneda,Kohei Tada,Harutaka Katano,Tetsutaro Sata
Neuropathology : official journal of the Japanese Society of Neuropathology
30
2010
Show Abstract
Human herpes virus 6 (HHV6) has attracted attention in recent years as an important causative agent for limbic encephalitis after bone marrow transplantation (BMT). We report an autopsy case of HHV6-induced encephalomyelitis that developed after BMT. The patient was a 61-year-old man with acute myeloid leukemia, who developed disorientation and short-term memory disturbance 35 days after allogenic BMT. MRI demonstrated T1-weighted high-signal intensity lesions in the medial temporal lobe and thalamus, and PCR of the CSF disclosed an increase in the copy numbers of the HHV6 genome. The patient died after a clinical course of 6 months, and at autopsy the brain showed remarkable atrophy of the hippocampus. Histopathologically, neuronal loss with astrocytosis and patchy necrosis with infiltration of macrophages were found predominantly in the hippocampus, amygdala, mamillary body, claustrum, and thalamus. Perivascular and intraparenchymal lymphocytic infiltration was slight. Similar lesions were also scattered in the cerebral neocortex, midbrain, pontine base, cerebellar white matter, and lumbar cord. In some of these lesions, axons were relatively preserved in comparison with myelin sheaths. Significant increase in the copy numbers of the HHV6 genome was demonstrated in the postmortem brain tissue by PCR. Neuropathological features of the present case were similar to those described in previously reported cases, but the distribution of lesions was more widespread. Demyelination was supposed to be involved in the pathogenesis of some of the lesions. | 19422536
 |
Quantitative HHV-6B antigenemia test for the monitoring of transplant patients.
R Loginov,T Karlsson,K Höckerstedt,D Ablashi,I Lautenschlager
European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology
29
2010
Show Abstract
Human herpesvirus-6 (HHV-6) infection, mostly caused by variant B, is common after transplantation. Here, we report a new modified method using an HHV-6B glycoprotein IgG antibody, OHV-3, and attempt to quantify the HHV-6 antigenemia after liver transplantation. Twenty-four liver transplant recipients were frequently monitored by the HHV-6 antigenemia test, which detects the HHV-6B virion protein in peripheral blood mononuclear cells (PBMC). HHV-6B antigens were now retrospectively demonstrated using a glycoprotein OHV-3 IgG antibody in the immunoperoxidase staining from the same specimens and quantified as positive cells/10,000 PBMC. The results were confirmed and quantified by DNA hybridization in situ. Altogether, 206 blood specimens were analyzed. During the first six months, HHV-6 antigenemia was detected in 17/24 (71%) recipients by using the HHV-6B virion antibody. In total, 37% (77/206) of specimens were positive with the virion antibody and 39% (78/201) by the OHV-3 antibody. The peak number of OHV-3-positive cells in the PBMC varied from 5 to 750/10,000 (mean 140/10,000). The OHV-3 antibody was useful to quantify the HHV-6B antigenemia. The findings of the HHV-6B quantitative antigenemia using the OHV-3 antibody correlated well with the previous qualitative HHV-6 antigenemia assay, and can be used as an alternative quantitative method in the monitoring of HHV-6 in transplant patients. | 20407819
 |
Human herpesvirus-7 in Brazilian liver transplant recipients: a follow-up comparison between molecular and immunological assays.
M F Peigo,R L Thomasini,A L P Puglia,S C B Costa,S H A Bonon,I F S Boin,M Leonardi,N G S Mota
Transplant infectious disease : an official journal of the Transplantation Society
11
2009
Show Abstract
Human herpesvirus-6 and -7 (HHV-6, HHV-7) remain latent after primary infection and can reactivate after transplantation. HHV-6 active infection has been related to some clinical manifestation, but the role of HHV-7 remains unclear. The clinical significance of HHV-7 DNAemia is not completely known and the immune response against HHV-7 has been poorly studied in transplantation. In this study, we investigated HHV-7 DNAemia in liver transplant recipients and evaluated the immunoglobulin (Ig) G and IgM response against HHV-7. A total of 22 adult liver transplant recipients were followed up for 90 days. HHV-7 DNAemia was detected by nested polymerase chain reaction (PCR) in DNA extracted from sera. IgG and IgM detection was performed by immunofluorescent assay using HHV-7-infected cord blood mononuclear cells. A significant virus antibody response was defined as either a positive IgM or a > or =4-fold rise in the virus IgG antibody. All patients had pre-transplant HHV-7-positive serostatus. Nine of 22 (40.9%) patients presented HHV-7 DNAemia during follow-up. All these patients had anti-HHV-7-positive IgM and/or significant increase in IgG titers with concurrent or subsequent DNAemia. In patients without DNAemia and low persistent IgG antibody titers, IgM was not detected. Correlation between nested PCR and IgM detection was statistically significant (P=0.01). Our study indicates that nested PCR in DNA extraction from serum can be useful to detect and monitor HHV-7 active infection in liver transplant recipients. IgM antibody detection also can be useful as a first immunological technique to detect active infection, especially if combined with PCR. | 19671120
 |