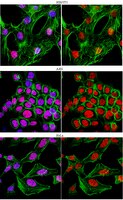
Critical Function of γH2A in S-Phase.
Mejia-Ramirez, E; Limbo, O; Langerak, P; Russell, P
PLoS genetics
11
e1005517
2015
Show Abstract
Phosphorylation of histone H2AX by ATM and ATR establishes a chromatin recruitment platform for DNA damage response proteins. Phospho-H2AX (γH2AX) has been most intensively studied in the context of DNA double-strand breaks caused by exogenous clastogens, but recent studies suggest that DNA replication stress also triggers formation of γH2A (ortholog of γH2AX) in Schizosaccharomyces pombe. Here, a focused genetic screen in fission yeast reveals that γH2A is critical when there are defects in Replication Factor C (RFC), which loads proliferating cell nuclear antigen (PCNA) clamp onto duplex DNA. Surprisingly Chk1, Cds1/Chk2 and the Rad9-Hus1-Rad1 checkpoint clamp, which are crucial for surviving many genotoxins, are fully dispensable in RFC-defective cells. Immunoblot analysis confirms that Rad9-Hus1-Rad1 is not required for formation of γH2A by Rad3/ATR in S-phase. Defects in DNA polymerase epsilon, which binds PCNA in the replisome, also create an acute need for γH2A. These requirements for γH2A were traced to its role in docking with Brc1, which is a 6-BRCT-domain protein that is structurally related to budding yeast Rtt107 and mammalian PTIP. Brc1, which localizes at stalled replication forks by binding γH2A, prevents aberrant formation of Replication Protein A (RPA) foci in RFC-impaired cells, suggesting that Brc1-coated chromatin stabilizes replisomes when PCNA or DNA polymerase availability limits DNA synthesis. | | | 26368543
 |
Mutation of histone H3 serine 86 disrupts GATA factor Ams2 expression and precise chromosome segregation in fission yeast.
Lim, KK; Ong, TY; Tan, YR; Yang, EG; Ren, B; Seah, KS; Yang, Z; Tan, TS; Dymock, BW; Chen, ES
Scientific reports
5
14064
2015
Show Abstract
Eukaryotic genomes are packed into discrete units, referred to as nucleosomes, by organizing around scaffolding histone proteins. The interplay between these histones and the DNA can dynamically regulate the function of the chromosomal domain. Here, we interrogated the function of a pair of juxtaposing serine residues (S86 and S87) that reside within the histone fold of histone H3. We show that fission yeast cells expressing a mutant histone H3 disrupted at S86 and S87 (hht2-S86AS87A) exhibited unequal chromosome segregation, disrupted transcriptional silencing of centromeric chromatin, and reduced expression of Ams2, a GATA-factor that regulates localization of the centromere-specific histone H3 variant CENP-A. We found that overexpression of ams2(+) could suppress the chromosome missegregation phenotype that arose in the hht2-S86AS87A mutant. We further demonstrate that centromeric localization of SpCENP-A(cnp1-1) was significantly compromised in hht2-S86AS87A, suggesting synergism between histone H3 and the centromere-targeting domain of SpCENP-A. Taken together, our work presents evidence for an uncharacterized serine residue in fission yeast histone H3 that affects centromeric integrity via regulating the expression of the SpCENP-A-localizing Ams2 protein. [173/200 words]. | | | 26369364
 |
Bub1 autophosphorylation feeds back to regulate kinetochore docking and promote localized substrate phosphorylation.
Asghar, A; Lajeunesse, A; Dulla, K; Combes, G; Thebault, P; Nigg, EA; Elowe, S
Nature communications
6
8364
2015
Show Abstract
During mitosis, Bub1 kinase phosphorylates histone H2A-T120 to promote centromere sister chromatid cohesion through recruitment of shugoshin (Sgo) proteins. The regulation and dynamics of H2A-T120 phosphorylation are poorly understood. Using quantitative phosphoproteomics we show that Bub1 is autophosphorylated at numerous sites. We confirm mitosis-specific autophosphorylation of a several residues and show that Bub1 activation is primed in interphase but fully achieved only in mitosis. Mutation of a single autophosphorylation site T589 alters kinetochore turnover of Bub1 and results in uniform H2A-T120 phosphorylation and Sgo recruitment along chromosome arms. Consequently, improper sister chromatid resolution and chromosome segregation errors are observed. Kinetochore tethering of Bub1-T589A refocuses H2A-T120 phosphorylation and Sgo1 to centromeres. Recruitment of the Bub1-Bub3-BubR1 axis to kinetochores has recently been extensively studied. Our data provide novel insight into the regulation and kinetochore residency of Bub1 and indicate that its localization is dynamic and tightly controlled through feedback autophosphorylation. | | | 26399325
 |
The EBNA3 family of Epstein-Barr virus nuclear proteins associates with the USP46/USP12 deubiquitination complexes to regulate lymphoblastoid cell line growth.
Ohashi, M; Holthaus, AM; Calderwood, MA; Lai, CY; Krastins, B; Sarracino, D; Johannsen, E
PLoS pathogens
11
e1004822
2015
Show Abstract
The Epstein-Barr virus (EBV) nuclear proteins EBNA3A, EBNA3B, and EBNA3C interact with the cell DNA binding protein RBPJ and regulate cell and viral genes. Repression of the CDKN2A tumor suppressor gene products p16(INK4A) and p14(ARF) by EBNA3A and EBNA3C is critical for EBV mediated transformation of resting B lymphocytes into immortalized lymphoblastoid cell lines (LCLs). To define the composition of endogenous EBNA3 protein complexes, we generated lymphoblastoid cell lines (LCLs) expressing flag-HA tagged EBNA3A, EBNA3B, or EBNA3C and used tandem affinity purification to isolate each EBNA3 complex. Our results demonstrated that each EBNA3 protein forms a distinct complex with RBPJ. Mass-spectrometry revealed that the EBNA3A and EBNA3B complexes also contained the deubquitylation complex consisting of WDR48, WDR20, and USP46 (or its paralog USP12) and that EBNA3C complexes contained WDR48. Immunoprecipitation confirmed that EBNA3A, EBNA3B, and EBNA3C association with the USP46 complex. Using chromatin immunoprecipitation, we demonstrate that WDR48 and USP46 are recruited to the p14(ARF) promoter in an EBNA3C dependent manner. Mapping studies were consistent with WDR48 being the primary mediator of EBNA3 association with the DUB complex. By ChIP assay, WDR48 was recruited to the p14(ARF) promoter in an EBNA3C dependent manner. Importantly, WDR48 associated with EBNA3A and EBNA3C domains that are critical for LCL growth, suggesting a role for USP46/USP12 in EBV induced growth transformation. | | | 25855980
 |
The nucleosome acidic patch plays a critical role in RNF168-dependent ubiquitination of histone H2A.
Mattiroli, F; Uckelmann, M; Sahtoe, DD; van Dijk, WJ; Sixma, TK
Nature communications
5
3291
2014
Show Abstract
During DNA damage response, the RING E3 ligase RNF168 ubiquitinates nucleosomal H2A at K13-15. Here we show that the ubiquitination reaction is regulated by its substrate. We define a region on the RING domain important for target recognition and identify the H2A/H2B dimer as the minimal substrate to confer lysine specificity to the RNF168 reaction. Importantly, we find an active role for the substrate in the reaction. H2A/H2B dimers and nucleosomes enhance the E3-mediated discharge of ubiquitin from the E2 and redirect the reaction towards the relevant target, in a process that depends on an intact acidic patch. This active contribution of a region distal from the target lysine provides regulation of the specific K13-15 ubiquitination reaction during the complex signalling process at DNA damage sites. | Western Blotting | | 24518117
 |
The histone H2A deubiquitinase Usp16 regulates embryonic stem cell gene expression and lineage commitment.
Yang, W; Lee, YH; Jones, AE; Woolnough, JL; Zhou, D; Dai, Q; Wu, Q; Giles, KE; Townes, TM; Wang, H
Nature communications
5
3818
2014
Show Abstract
Polycomb Repressive Complex 1 and histone H2A ubiquitination (ubH2A) contribute to embryonic stem cell (ESC) pluripotency by repressing lineage-specific gene expression. However, whether active deubiquitination co-regulates ubH2A levels in ESCs and during differentiation is not known. Here we report that Usp16, a histone H2A deubiquitinase, regulates H2A deubiquitination and gene expression in ESCs, and importantly, is required for ESC differentiation. Usp16 knockout is embryonic lethal in mice, but does not affect ESC viability or identity. Usp16 binds to the promoter regions of a large number of genes in ESCs, and Usp16 binding is inversely correlated with ubH2A levels, and positively correlates with gene expression levels. Intriguingly, Usp16(-/-) ESCs fail to differentiate due to ubH2A-mediated repression of lineage-specific genes. Finally, Usp16, but not a catalytically inactive mutant, rescues the differentiation defects of Usp16(-/-) ESCs. Therefore, this study identifies Usp16 and H2A deubiquitination as critical regulators of ESC gene expression and differentiation. | | | 24784029
 |
Uncoupling transcription from covalent histone modification.
Zhang, H; Gao, L; Anandhakumar, J; Gross, DS
PLoS genetics
10
e1004202
2014
Show Abstract
It is widely accepted that transcriptional regulation of eukaryotic genes is intimately coupled to covalent modifications of the underlying chromatin template, and in certain cases the functional consequences of these modifications have been characterized. Here we present evidence that gene activation in the silent heterochromatin of the yeast Saccharomyces cerevisiae can occur in the context of little, if any, covalent histone modification. Using a SIR-regulated heat shock-inducible transgene, hsp82-2001, and a natural drug-inducible subtelomeric gene, YFR057w, as models we demonstrate that substantial transcriptional induction (greater than 200-fold) can occur in the context of restricted histone loss and negligible levels of H3K4 trimethylation, H3K36 trimethylation and H3K79 dimethylation, modifications commonly linked to transcription initiation and elongation. Heterochromatic gene activation can also occur with minimal H3 and H4 lysine acetylation and without replacement of H2A with the transcription-linked variant H2A.Z. Importantly, absence of histone modification does not stem from reduced transcriptional output, since hsp82-ΔTATA, a euchromatic promoter mutant lacking a TATA box and with threefold lower induced transcription than heterochromatic hsp82-2001, is strongly hyperacetylated in response to heat shock. Consistent with negligible H3K79 dimethylation, dot1Δ cells lacking H3K79 methylase activity show unimpeded occupancy of RNA polymerase II within activated heterochromatic promoter and coding regions. Our results indicate that large increases in transcription can be observed in the virtual absence of histone modifications often thought necessary for gene activation. | | | 24722509
 |
Histone content increases in differentiating embryonic stem cells.
Karnavas, T; Pintonello, L; Agresti, A; Bianchi, ME
Frontiers in physiology
5
330
2014
Show Abstract
Mouse Embryonic Stem Cells (ESCs) are pluripotent mammalian cells derived from the Inner Cell Mass (ICM) of mouse blastocysts, which give rise to all three embryonic germ layers both in vivo and in vitro. Mouse ESCs have a distinct epigenetic landscape and a more decondensed chromatin compared to differentiated cells. Numerous studies have shown that distinct histone modifications in ESCs serve as hallmarks of pluripotency. However, so far it is still unknown whether the total histone content (as opposed to histone modifications) remains the same in cells of different developmental stage and differentiation capacity. In this work we show that total histone content differs between pluripotent and differentiated cells. In vitro spontaneous differentiation from ESCs to Embryoid Bodies (EBs) and directed differentiation toward neuronal and endodermal cells entails an increase in histone content. Primary MEFs also contain more histones than ESCs. We suggest that the difference in histone content is an additional hallmark of pluripotency, in addition to and besides histone modifications. | Western Blotting | | 25221520
 |
Phosphorylation and arginine methylation mark histone H2A prior to deposition during Xenopus laevis development.
Wang, WL; Anderson, LC; Nicklay, JJ; Chen, H; Gamble, MJ; Shabanowitz, J; Hunt, DF; Shechter, D
Epigenetics & chromatin
7
22
2014
Show Abstract
Stored, soluble histones in eggs are essential for early development, in particular during the maternally controlled early cell cycles in the absence of transcription. Histone post-translational modifications (PTMs) direct and regulate chromatin-templated transactions, so understanding the nature and function of pre-deposition maternal histones is essential to deciphering mechanisms of regulation of development, chromatin assembly, and transcription. Little is known about histone H2A pre-deposition modifications nor known about the transitions that occur upon the onset of zygotic control of the cell cycle and transcription at the mid-blastula transition (MBT).We isolated histones from staged Xenopus laevis oocytes, eggs, embryos, and assembled pronuclei to identify changes in histone H2A modifications prior to deposition and in chromatin. Soluble and chromatin-bound histones from eggs and embryos demonstrated distinct patterns of maternal and zygotic H2A PTMs, with significant pre-deposition quantities of S1ph and R3me1, and R3me2s. We observed the first functional distinction between H2A and H4 S1 phosphorylation, as we showed that H2A and H2A.X-F (also known as H2A.X.3) serine 1 (S1) is phosphorylated concomitant with germinal vesicle breakdown (GVBD) while H4 serine 1 phosphorylation occurs post-MBT. In egg extract H2A/H4 S1 phosphorylation is independent of the cell cycle, chromatin assembly, and DNA replication. H2AS1ph is highly enriched on blastula chromatin during repression of zygotic gene expression while H4S1ph is correlated with the beginning of maternal gene expression and the lengthening of the cell cycle, consistent with distinct biological roles for H2A and H4 S1 phosphorylation. We isolated soluble H2A and H2A.X-F from the egg and chromatin-bound in pronuclei and analyzed them by mass spectrometry analysis to quantitatively determine abundances of S1ph and R3 methylation. We show that H2A and H4 S1ph, R3me1 and R3me2s are enriched on nucleosomes containing both active and repressive histone PTMs in human A549 cells and Xenopus embryos.Significantly, we demonstrated that H2A phosphorylation and H4 arginine methylation form a new class of bona fide pre-deposition modifications in the vertebrate embryo. We show that S1ph and R3me containing chromatin domains are not correlated with H3 regulatory PTMs, suggesting a unique role for phosphorylation and arginine methylation. | Immunoblotting (Western) | | 25302076
 |
Ataxia telangiectasia mutated (ATM) is dispensable for endonuclease I-SceI-induced homologous recombination in mouse embryonic stem cells.
Rass, E; Chandramouly, G; Zha, S; Alt, FW; Xie, A
The Journal of biological chemistry
288
7086-95
2013
Show Abstract
Ataxia telangiectasia mutated (ATM) is activated upon DNA double strand breaks (DSBs) and phosphorylates numerous DSB response proteins, including histone H2AX on serine 139 (Ser-139) to form γ-H2AX. Through interaction with MDC1, γ-H2AX promotes DSB repair by homologous recombination (HR). H2AX Ser-139 can also be phosphorylated by DNA-dependent protein kinase catalytic subunit and ataxia telangiectasia- and Rad3-related kinase. Thus, we tested whether ATM functions in HR, particularly that controlled by γ-H2AX, by comparing HR occurring at the euchromatic ROSA26 locus between mouse embryonic stem cells lacking either ATM, H2AX, or both. We show here that loss of ATM does not impair HR, including H2AX-dependent HR, but confers sensitivity to inhibition of poly(ADP-ribose) polymerases. Loss of ATM or H2AX has independent contributions to cellular sensitivity to ionizing radiation. The ATM-independent HR function of H2AX requires both Ser-139 phosphorylation and γ-H2AX/MDC1 interaction. Our data suggest that ATM is dispensable for HR, including that controlled by H2AX, in the context of euchromatin, excluding the implication of such an HR function in genomic instability, hypersensitivity to DNA damage, and poly(ADP-ribose) polymerase inhibition associated with ATM deficiency. | | | 23355489
 |